Caesium
Caesium (or cesium) is the chemical element with the atomic number 55 on the periodic table. Its symbol is Cs.
 | ||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈsiːziəm/ | |||||||||||||||||||||||||||
| Alternative name | cesium (US, informal) | |||||||||||||||||||||||||||
| Appearance | pale gold | |||||||||||||||||||||||||||
| Standard atomic weight (Ar, standard) | 132.90545196(6)[1] | |||||||||||||||||||||||||||
| Caesium in the periodic table | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Atomic number (Z) | 55 | |||||||||||||||||||||||||||
| Group | group 1 (alkali metals) | |||||||||||||||||||||||||||
| Period | period 6 | |||||||||||||||||||||||||||
| Block | s-block | |||||||||||||||||||||||||||
| Element category | alkali metal | |||||||||||||||||||||||||||
| Electron configuration | [Xe] 6s1 | |||||||||||||||||||||||||||
Electrons per shell | 2, 8, 18, 18, 8, 1 | |||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||
| Phase at STP | Cs: Solid | |||||||||||||||||||||||||||
| Melting point | 301.7 K (28.5 °C, 83.3 °F) | |||||||||||||||||||||||||||
| Boiling point | 944 K (671 °C, 1240 °F) | |||||||||||||||||||||||||||
| Density (near r.t.) | 1.93 g/cm3 | |||||||||||||||||||||||||||
| when liquid (at m.p.) | 1.843 g/cm3 | |||||||||||||||||||||||||||
| Critical point | 1938 K, 9.4 MPa[2] | |||||||||||||||||||||||||||
| Heat of fusion | 2.09 kJ/mol | |||||||||||||||||||||||||||
| Heat of vaporization | 63.9 kJ/mol | |||||||||||||||||||||||||||
| Molar heat capacity | 32.210 J/(mol·K) | |||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||
| Oxidation states | −1, +1[3] (a strongly basic oxide) | |||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.79 | |||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||
| Atomic radius | empirical: 265 pm | |||||||||||||||||||||||||||
| Covalent radius | 244±11 pm | |||||||||||||||||||||||||||
| Van der Waals radius | 343 pm | |||||||||||||||||||||||||||
| Spectral lines of caesium | ||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||
| Natural occurrence | Cs: Primordial | |||||||||||||||||||||||||||
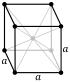
| Crystal structure | body-centered cubic (bcc) | |||||||||||||||||||||||||||
| Thermal expansion | 97 µm/(m·K) (at 25 °C) | |||||||||||||||||||||||||||
| Thermal conductivity | 35.9 W/(m·K) | |||||||||||||||||||||||||||
| Electrical resistivity | 205 nΩ·m (at 20 °C) | |||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[4] | |||||||||||||||||||||||||||
| Young's modulus | 1.7 GPa | |||||||||||||||||||||||||||
| Bulk modulus | 1.6 GPa | |||||||||||||||||||||||||||
| Mohs hardness | 0.2 | |||||||||||||||||||||||||||
| Brinell hardness | 0.14 MPa | |||||||||||||||||||||||||||
| CAS Number | 7440-46-2 | |||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||
| Naming | from Latin caesius, sky blue, for its spectral colours | |||||||||||||||||||||||||||
| Discovery | Robert Bunsen and Gustav Kirchhoff (1860) | |||||||||||||||||||||||||||
| First isolation | Carl Setterberg (1882) | |||||||||||||||||||||||||||
| Main isotopes of caesium | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
Caesium is an alkali metal. Its melting point is low (28 °C). It is extremely reactive. Because of its high reactivity, it is a dangerous chemical. It may set itself on fire (ignite) in air. It explodes on contact with water. It reacts more violently than the other alkali metals with water. Because of this, caesium is stored in mineral oil.[6]
Caesium is a rare element. Since there is little caesium on the Earth, it is rather expensive. The human body does not need caesium. In large amounts, its chemical compounds are mildly poisonous because it is close to potassium, which the body does need.
History
Caesium was first described in 1860, by Gustav Robert Kirchhoff and Robert Wilhelm Bunsen. They were testing mineral water, from Bad Dürkheim. After they separated calcium, strontium, magnesium and lithium, they saw two lines in the "blue" range of the spectrum. Because of these lines, they concluded that in addition to the elements already found, there must be another unknown substance in the mineral water. They named this substance caesium, after the color blue.[7]
Isotopes and compounds
Caesium has at least 39 known isotopes ranging in atomic mass from 112 to 151. Only one of these, 133Cs, is stable. Therefore, the naturally-occurring isotope of caesium is 133Cs, which is not radioactive. 133Cs is used in atomic clocks, its vibration frequency used to define the length of the second. Another isotope, 137Cs is not made naturally but is made after nuclear fission has been done. It is very radioactive and used as an industrial gamma ray source.
Caesium forms compounds with many other chemical elements. Caesium formate is used in oil drilling because of its high density.
Reactivity
Caesium is extremely reactive in air and water. Caesium rapidly oxidizes in air and can spontaneously combust[8] (randomly catch on fire) at any moment. For this reason, it must be stored in kerosene or a mineral oil, like other group one elements (Lithium, Natrium, Rubidium, and Francium.) In water, Caesium violently reacts to make Caesium Hydroxide (2CsOH).[9] The Caesium sinks for about one second, then explodes. The explosion is over 50 times the size of the element dropped in the water, and the explosion is enough to break a common Pyrex Beaker, Flask, or Test Tube. You can find a video of the reaction here.
Caesium Media
High-purity caesium-133 stored in argon.
Caesium crystals (golden) compared to rubidium crystals (silvery)
Addition of a small amount of caesium to cold water is explosive.
Monatomic caesium halide wires grown inside double-wall carbon nanotubes (TEM image).
References
- ↑ Meija, J.; Coplen, T. B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; Prohaska, T. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.121. ISBN 1439855110.
- ↑ 3.0 3.1 Dye, J. L. (1979). "Compounds of Alkali Metal Anions". Angewandte Chemie International Edition. 18 (8): 587–598. doi:10.1002/anie.197905871.
- ↑ "Magnetic susceptibility of the elements and inorganic compounds". Handbook of Chemistry and Physics (PDF) (87th ed.). CRC press. ISBN 0-8493-0487-3. Retrieved 2010-09-26.
- ↑ "NIST Radionuclide Half-Life Measurements". NIST. Retrieved 2011-03-13.
- ↑ William C. Butterman et al 2004. "Mineral Commodity Profile: Cesium" (PDF). United States Geological Survey. Retrieved 2009-12-27.
- ↑ G. Kirchhoff, R. Bunsen: Chemische Analyse durch Spectralbeobachtungen. In: Annalen der Physik und Chemie. 1861, 189, 7, S. 337–381 ().
- ↑ Interest, Compound (2019-08-06). "IYPT 2019 Elements 055: Caesium: Atomic clocks and explosive reactions". Compound Interest. Retrieved 2019-11-13.
- ↑ "WebElements Periodic Table » Caesium » reactions of elements". www.webelements.com. Retrieved 2019-11-13.