Alanine
Alanine (abbreviated as Ala or A)[3] is an α-amino acid. It has the chemical formula CH3CH(NH2)COOH.
| Alanine | |
|---|---|
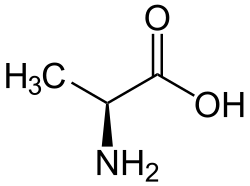
| |
| IUPAC name | Alanine |
| Other names | 2-Aminopropanoic acid |
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | C01401 |
| ChEBI | CHEBI:16977 |
| SMILES | O=C(O)C(N)C |
| Properties | |
| Molecular formula | C3H7NO2 |
| Molar mass | 89.08 g mol-1 |
| Appearance | white powder |
| Density | 1.424 g/cm3 |
| Melting point |
258 °C, 531 K, 496 °F |
| Solubility in water | 167.2 g/L (25 °C) |
| log P | -0.68[1] |
| Acidity (pKa) |
|
| -50.5·10−6 cm3/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a non-polar amino acid.[4]
L-Alanine accounts for 7.8% of the primary structure in a sample of 1,150 proteins.[5][6] Leucine is the only amino acid which is more common.
D-Alanine occurs in bacterial cell walls and in some peptide antibiotics.
Alanine Media
References
- ↑ "L-alanine_msds".
- ↑ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 5–88. ISBN 978-1498754286.
- ↑ Nomenclature and symbolism for amino acids and peptides. IUPAC-IUB Recommendations 1983. Pure Appl. Chem. 56 (5), 1984: 595–624.
- ↑ Non-polar: electrons shared so that there is no difference in electric charge between different parts of the molecule.
- ↑ Doolittle, R. F. (1989), "Redundancies in protein sequences", in Fasman, G. D. (ed.), Prediction of protein structures and the principles of protein conformation, New York: Plenum, pp. 599–623, ISBN 0-306-43131-9
- ↑ Primary structure: the exact specification of its atomic structure and its chemical bonds.


