Counterion
A counterion is an ion (a chemical compound with electrical charge) that is used to balance out the charge on a different ion in order to make a salt or solution. Counterions are needed because ions cannot exist on their own in liquid or solid form.
In sodium chloride, Na+
is the counterion of Cl−
, and Cl−
is the counterion of Na+
.
Counterion Media
Lithium tetrakis(pentafluorophenyl)borate is the lithium salt of a highly lipophilic tetraarylborate anion, often referred to as a weakly coordinating anion.
Tetraphenylborate is less lipophilic than the perfluorinated derivative, but widely used as a precipitating agent.
Hexafluorophosphate is a common weakly coordinating anion.
As illustrated by the small counteranion tetrafluoroborate (BF−
4), lipophilic cations tend to be symmetric and singly charged.Bis(triphenylphosphine)iminium chloride is the chloride salt of a bulky lipophilic phosphonium cation [Ph3PNPPh3]+.
Tetraphenylphosphonium chloride (C6H5)4PCl, abbreviated Ph4PCl or PPh4Cl is the chloride of a symmetrical phosphonium cation that is often used in organometallic chemistry. The arsonium salt is also well known.
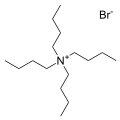
The bromide salt of tetrabutylammonium, one of the most common counter cations. Many analogous "quat salts" are known.
Alkali metal cations bound by crown ethers are common lipophilic countercations, as illustrated by [Li(12-crown-4)2]+.