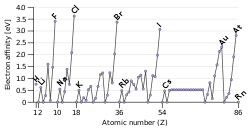Electron affinity
Electron affinity is a phrase used in chemistry. When an atom that is neutral gets an electron, the energy that is changed in the atom is the electron affinity. Across a period, the electron affinity increases. [1]
Electron Affinity Media
Band diagram of semiconductor-vacuum interface showing electron affinity EEA, defined as the difference between near-surface vacuum energy Evac, and near-surface conduction band edge EC. Also shown: Fermi level EF, valence band edge EV, work function W.
References
- ↑ Davis, Raymond E., Regina Frey, and Mickey Sarquis. Modern Chemistry. Holt, Rhinehart, and Wintston. Print.