Epoxy
Epoxy is the end product of epoxy resins.
It is also a common name for the epoxide functional group, and a common name for a type of strong adhesive used for sticking things together.[1] Typically, two resins need to be mixed together before use.
Epoxy resins are a class of reactive polymers which contain epoxide groups. Their co-reactants are often called 'hardeners' or 'curatives', and the cross-linking reaction is referred to as 'curing'. The reaction forms a thermosetting polymer, often with strong mechanical properties as well as high temperature and chemical resistance. Epoxy has a wide range of industrial applications.
- Superglue is another strong adhesive, made of cyanoacrylate.
Epoxy Media
Structure of the epoxide group, a reactive functional group present in all epoxy resins
Structure of a cured epoxy glue. The triamine hardener is shown in red, the resin in black. The resin's epoxide groups have reacted with the hardener and are not present anymore. The material is highly crosslinked and contains many OH groups, which confer adhesive properties
Structure of TETA, a typical hardener. The amine (NH2) groups react with the epoxide groups of the resin during polymerisation.
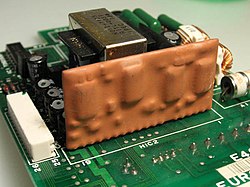
An epoxy encapsulated hybrid circuit on a printed circuit board.
Notes
- ↑ Definition of epoxy resin, noun, from the Cambridge Advanced Learner's Dictionary & Thesaurus, Cambridge University Press.
+{{{1}}}−{{{2}}}