Mineralogy
Mineralogy is the study of minerals. Minerals are things that make rocks. There are many different types of minerals. Some are hard, like diamonds. Some are soft, like talc. Some are metal, like gold or silver. Minerals are put into special groups of minerals made of similar chemicals, or that have similar structures inside. For example, the chemicals that make up some minerals line up in chains, while in other minerals, they make bow-tie shapes.
Studying minerals can be useful for figuring out certain things about a rock. Sometimes the shape or size of the mineral can tell something about the rock as well. For example, minerals in igneous rocks can help figure out how long the rock took to cool down (turn from lava into a rock). Larger minerals mean the rock cooled slowly (probably underground). Smaller minerals mean the rock cooled down quickly (probably above ground, like from a volcano). The type of mineral in a rock can also tell what kind of a rock it is, or what has happened to the rock since it formed. Many rocks are named based on what kinds of minerals they have.
Mineralogists (people who study minerals) study minerals in rocks with hand lenses (a magnifying glass), and in thin section (thin slices of rock) with microscopes. They record things about the minerals like how big they are, what shape they are, what color they are, and if the mineral changes colors when you turn it. Mineralogists also record what color minerals turn in a special light. These details can help mineralogists figure out what minerals they are looking at.
Minerals can be used in a lot of different things, like mining, jewelry, farming, pottery, making metals, and more . Mineralogists can help find important minerals in the Earth using what they know and learn.
Mineralogy Media
Mineralogy applies principles of chemistry, geology, physics and materials science to the study of minerals
Page from Treatise on mineralogy by Friedrich Mohs (1825)
The Moon Mineralogy Mapper, a spectrometer that mapped the lunar surface
Calcite is a carbonate mineral (CaCO3) with a rhombohedral crystal structure.
Aragonite is an orthorhombic polymorph of calcite.
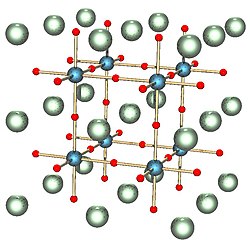
The perovskite crystal structure. The most abundant mineral in the Earth, bridgmanite, has this structure. Its chemical formula is (Mg,Fe)SiO3; the red spheres are oxygen, the blue spheres silicon and the green spheres magnesium or iron.
Photomicrograph of olivine adcumulate from the Archaean komatiite of Agnew, Western Australia.
Hanksite, Na22K(SO4)9(CO3)2Cl, one of the few minerals that is considered a carbonate and a sulfate
Other websites
- International Mineralogical Association
- Mineralogical Association of Canada
- Virtual Museum of the History of Mineralogy Archived 2010-04-28 at the Wayback Machine
- The Giant Crystal Project Archived 2008-04-26 at the Wayback Machine
- The Geological Society of America Archived 2011-10-29 at the Wayback Machine