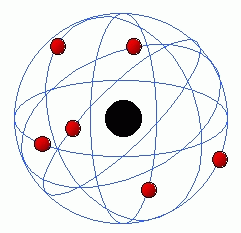
Rutherford model
the rutherford model is a model by ernst rutherford.[1] it was created by a experment that use the gold foil for alpha partcles.[2] it said that that thomson's plum pudding model was wrong and his model was better then his model.[3] his model was however also wrong and the bohr model came after his model.[4]
bohr model
bohr model is a fixed version of rutherford model and it said model existed that electrons different energy level but original rutherford model didnt say that and instead said model like planets around sun.[5]
Rutherford Model Media
Schematic diagram Rutherford's atom: electrons in green and nucleus in red. The atomic nucleus shown expanded more than 10,000 times its size relative to the atom; electrons have no measurable diameter.
3D animation of an atom incorporating the Rutherford model. The atomic nucleus shown expanded more than 10,000 times its size relative to the atom; electrons have no measurable diameter.
- ↑ https://www.britannica.com/science/atom
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Chemistry_for_Changing_Times_(Hill_and_McCreary)/03%3A_Atomic_Structure/3.04%3A_Rutherford's_Experiment-_The_Nuclear_Model_of_the_Atom#:~:text=Rutherford's%20atomic%20model%20became%20known,the%20volume%20of%20the%20atom.
- ↑ https://www.vedantu.com/question-answer/why-was-j-j-thomson-wrong-class-11-chemistry-cbse-6112949aa947c07fce1f5f40
- ↑ https://homework.study.com/explanation/was-rutherford-s-model-of-the-atom-correct.html#:~:text=Answer%20and%20Explanation%3A,single%2C%20positively%2Dcharged%20structure.
- ↑ https://www.britannica.com/science/Rutherford-model#:~:text=Niels%20Bohr%20built%20upon%20Rutherford's,the%20problem%20of%20atomic%20structure.