Solution (chemistry)
In chemistry, a solution is a homogeneous mixture of two or more substances. The substances that are dissolved are called solutes.[1] The substance the solutes are dissolved in is called the solvent. An example from everyday experience is a solid like salt or sugar (which are crystalline solids), dissolved in a liquid (like water). Gases can dissolve in liquids. An example is carbon dioxide or oxygen in water. Liquids may dissolve in other liquids and gases in other gases.
The amount of solute added to the solvent determines the concentration of the solution. The solution with the large amount of solute is called a concentrated solution; the solution with less solute is called a dilute solution.
Examples of solid solutions are alloys and some minerals. For example, brass is an alloy of copper and zinc.
An aqueous solution is one that contains water as the solvent.[2] An unsaturated solution has less solute than it can dissolve, while a saturated solution contains the maximum amount of solute it can hold.[2] A supersaturated solution holds more solute than is typically possible under normal conditions.[2]
Solution (chemistry) Media
Making a saline water solution by dissolving table salt (NaCl) in water. The salt is the solute and the water the solvent.
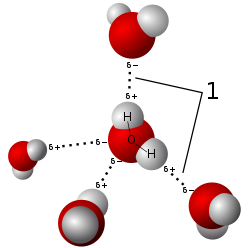
Water is a good solvent for some polar materials because water molecules are polar and capable of forming hydrogen bonds.
Related pages
References
- ↑ "Solution". Merriam-Webster dictionary. Archived from the original on 2007-04-04. Retrieved 2007-06-29.
- ↑ 2.0 2.1 2.2 Kavanah, Patrick (2018). Chemistry: The Physical Setting. Pearson. ISBN 978-0-328-98858-7.