Absolute temperature
Absolute temperature, also called thermodynamic temperature, is the temperature of an object on a scale where zero is taken as absolute zero. Absolute temperature scales are Kelvin and Rankine.
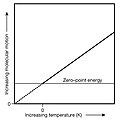
Absolute zero is the temperature at which a system is in the state of lowest possible (minimum) energy. As molecules approach this temperature, their movements continue to slow down. The kinetic energy of the molecules becomes negligible. However, the molecules will never stop moving nor have no energy.
It is the lowest temperature a gas thermometer can measure. No electronic devices work at this temperature. No living thing can survive in this temperature.
Examples
Common temperatures in the absolute scale are:
- 0 °C (freezing point of water) = 273.15 K
- 25 °C (room temperature) = 298.15 K
- 100 °C (boiling point of water) = 373.15 K
- 0 K (absolute zero) = -273.15 °C
- 233.15 K (equal measures in Celsius and Fahrenheit)= -40 Celsius
- Triple point of water= 273.16K (equal measure in Celsius) 0.01°C.
Conversion
To convert from the Celsius scale into the absolute temperature, you add 273.15 and change °C to K. To get a temperature on the absolute scale to the Celsius scale, subtract 273.15 and change K to °C. This is normally used in the science world. Kelvin is used globally as a part of the International System of Units. It is one of the 7 base units of the system. The value of absolute temperature is 0 K.
- Celsius to Kelvin: K = C + 273.15
- Kelvin to Celsius: C = K - 273.15
- Fahrenheit to Rankine: R = F + 459.67
- Rankine to Fahrenheit: F = R - 459.67
Absolute Temperature Media
Figure 3 Molecules have internal structures because they are composed of atoms that have different ways of moving within molecules. Being able to store kinetic energy in these internal degrees of freedom contributes to a substance's specific heat capacity, or internal energy, allowing it to contain more internal energy at the same temperature.
Figure 4 The temperature-induced translational motion of particles in solids takes the form of phonons. Shown here are phonons with identical amplitudes but with wavelengths ranging from 2 to 12 average inter-molecule separations (a).
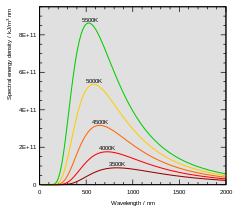
Figure 5 The spectrum of black-body radiation has the form of a Planck curve. A 5500 K black-body has a peak emittance wavelength of 527 nm. Compare the shape of this curve to that of a Maxwell distribution in Fig. 2 above.
Figure 8 When many of the chemical elements, such as the noble gases and platinum-group metals, freeze to a solid — the most ordered state of matter — their crystal structures have a close-packed arrangement. This yields the greatest possible packing density and the lowest energy state.