Amine
| Primary amine | Secondary amine | Tertiary amine |
|---|---|---|
 |
 |
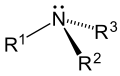 |
An amine is a molecule which has a nitrogen atom that can behave as a base. An amine can be thought of coming from ammonia. The difference is that the three hydrogens in ammonia are changed to any group or atom. If only one of the hydrogens is changed, the amine is called primary. If two are changed, the amine is called secondary. If all three are changed then it is called tertiary.
Sometimes, a fourth group can be added to the nitrogen. This is called a quaternary ammonium cation. This is not an amine but is a salt. If one of the groups on the nitrogen is a carbonyl group, then the molecule is called an amide. This has very different properties.
Amines are used a lot in chemistry. Since the nitrogen has a lone pair, it can do many reactions. It can take protons away from some acids. It can also be a nucleophile. It can be changed into imines and other functional groups.
Amines are also found in many proteins. They are a part of every amino acid.[1]
Amine Media
2-aminobutane (or butan-2-amine)
Inversion of an amine spatial configuration: Amine "flip-flop" like an umbrella turned over by the wind. The pair of dots represents the lone electron pair on the nitrogen atom.
- Amide formation from amine.gif.
References
- ↑ "Amines - Formula, Structure, Nomenclature, Classification, Preparation, Basicity, FAQs and Videos of Amines". BYJUS. Retrieved 2023-09-13.


