Amide
Amides are two related groups of chemical compounds, both derived from ammonia and amines.[1]
Organic amides
In organic chemistry, amides are related to carboxylic acids. Organic amides are also called carboxamides.[2]
Replacing the hydroxyl group –OH of a carboyxlic acid with an amino group –NH
2 gives the amide of that acid: for example, acetic acid CH
3COOH becomes acetamide CH
3CONH
2.
One or more of the hydrogens on the amino group can also be replaced with other organic groups. If more than one acyl group is connected to the nitrogen atom, the amide can be called a secondary or tertiary amide.
Inorganic amides
Inorganic amides have inorganic cations bonded to the nitrogen atom of an amine. This bond can be covalent or ionic. The NH−
2 ion is also referred to as "amide" or "azanide", and is made by deprotonating ammonia with a strong base. This is ammonia acting as an acid, although a very weak one.
Because ammonia is a weak acid, inorganic amides are strong bases. Sodium amide is an example of an inorganic amide.
Amide Media
General structure of an amide (specifically, a carboxamide)
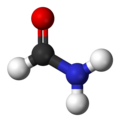
Formamide, the simplest amide
Asparagine (zwitterionic form), an amino acid with a side chain (highlighted) containing an amide group
Structure of acetamide hydrogen-bonded dimer from X-ray crystallography. Selected distances: C-O: 1.243, C-N, 1.325, N---O, 2.925 Å. Color code: red = O, blue = N, gray = C, white = H.
- Formylation of Benzene using Phenyllithium and DMF.png
Formylation of Benzene using Phenyllithium and DMF
- Acid-CatAmideHydrolMarch.png
Mechanism for acid-mediated hydrolysis of an amide.
- Bodroux reaction.svg
Bodroux reaction for synthesis of amide.
Related pages
References
- ↑ International Union of Pure and Applied Chemistry. "Amides". Compendium of Chemical Terminology Internet edition.
- ↑ International Union of Pure and Applied Chemistry. "Carboxamides". Compendium of Chemical Terminology Internet edition.