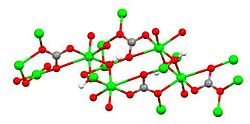
Copper(II) hydroxide
Copper(II) hydroxide, also known as cupric hydroxide, is a chemical compound. Its chemical formula is Cu(OH)2. It contains copper in its +2 oxidation state. It also contains hydroxide ions.
Properties
Copper(II) hydroxide is light blue. It is rubbery when wet. When it is mixed with copper(II) carbonate, it is greenish. It reacts with carbon dioxide to make copper(II) carbonate when in air. It turns into copper(II) oxide when wet. When it is dry, though, it only turns into copper(II) oxide when it is heated. It reacts with ammonia to make a very dark blue solution.
Preparation
Copper(II) hydroxide can be made by reacting copper sulfate with sodium hydroxide. Potassium hydroxide can be used, but it is more expensive. It can also be made by electrolyzing a solution of sodium bicarbonate with a copper anode.
Uses
Copper(II) hydroxide is used to kill mold in paints. It can be used to color ceramics. It can be used as a catalyst.
Safety
It is not toxic in small amounts, but it can dissolve in stomach acid to make soluble copper, which can poison much more easily.
Copper(II) Hydroxide Media
Chemical structure of azurite, one of many copper(II) hydroxides (color code: red = O, green = Cu, gray = C, white = H).