Diastereomer
| Diastereomers | |
|---|---|

|

|
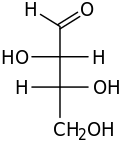
| D-Threose | D-Erythrose |
Diastereomers (also called diastereoisomers) are stereoisomers which are not enantiomers. They are molecules which have the same atoms and bonds but different stereochemistry in at least one, but not all, of their chiral centres. Diastereomers can have very different properties even though they look similar.[1] This is different from enantiomers, that have exactly the same properties except when in a chiral environment like the human body.
Every chiral centre in a molecule has two possible ways of putting the groups in space. With only one chiral centre, there are two enantiomers. With two chiral centres, you can form 4 different isomers. When all chiral centres are changed at the same time, two pairs of enantiomers are formed. When only one chiral centre is changed at a time, sets of diastereoisomers are formed. With more chiral centres the possibilities are many more.
Diastereoisomers which are different at only one of more chiral centres are called epimers. For example, D-Threose and D-Erythrose (see picture) are diastereomers because they are different at only one of two chiral centres. Because of this they can also be called epimers.[2]
Diastereomer Media
References
- ↑ "IUPAC Gold Book - Diastereoisomerism". IUPAC. Retrieved 22 January 2013.
- ↑ "IUPAC Gold Book - Epimers". IUPAC. Retrieved 22 January 2013.