Energy profile

Energy profiles of a reaction, with and without a catalyst. This reaction is endergonic because the products have more energy than the reactants. There is a single transition state and no intermediates.
An energy profile is a way of drawing a chemical reaction that shows how the energy changes as the reaction happens. The energy profile is a curve where the local maxima and minima represent transition states and reaction intermediates.
Energy Profile Media
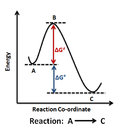
Figure 1: Reaction Coordinate Diagram: Starting material or reactant A convert to product C via the transition state B, with the help of activation energy ΔG≠, after which chemical energy ΔG° is released
Figure 3: PES for water molecule:Shows the energy minimum corresponding to optimized molecular structure for water, O–H bond length of 0.0958 nm and H–O–H bond angle of 104.5°