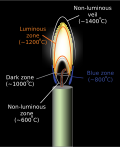
Flame
A flame is the visible part of a fire. It gives light and heat. It is the result of an exothermic reaction. The color and temperature of a flame depend on the type of fuel that is used to make the fire. A blue or white flame is often very hot, while a red, orange, or yellow flame is less hot.
Flame Media
Spectrum of the blue (premixed, i.e., complete combustion) flame from a butane torch showing molecular radical band emission and Swan bands. Virtually all the light produced is in the blue to green region of the spectrum below about 565 nanometers, accounting for the bluish color of sootless hydrocarbon flames.
Different flame types of a Bunsen burner depend on oxygen supply. On the left a rich fuel with no premixed oxygen produces a yellow sooty diffusion flame; on the right a lean fully oxygen premixed flame produces no soot and the flame color is produced by molecular radicals, especially CH and C2 band emission.
A flame test for sodium. The yellow color in this gas flame does not arise from the black-body emission of soot particles (as the flame is clearly a blue premixed complete combustion flame) but instead comes from the spectral line emission of sodium atoms, specifically the very intense sodium D lines.
In zero-G, convection does not carry the hot combustion products away from the fuel source, resulting in a spherical flame front.