Intermolecular force
In physics, chemistry, and biology, intermolecular forces are forces that act between stable molecules or between functional groups of macromolecules.
These forces are generally much weaker than the chemical bonding forces. Their bonding energies are less than a few kcal/mol. But they are responsible for many different physical, chemical, and biological phenomena. In general one distinguishes short and long range intermolecular forces. (The molecule is a substance are held together by the forces acting between the molecules which are called inter-molecular forces)
Hydrogen bonding
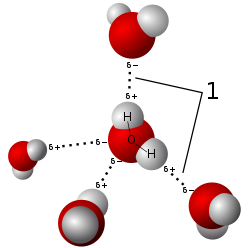
Hydrogen bonding is an intermolecular interaction with a hydrogen atom being present in the intermolecular bond.

Intermolecular Force Media
3D model of hydrogen bonding between water molecules, an example of a intermolecular force
Related pages
Other websites
Software for calculation of intermolecular forces
- Quantum 3.2 Archived 2017-09-14 at the Wayback Machine
- SAPT: An ab initio quantumchemical package.
}}