Neptunium

Neptunium is a chemical element that has the symbol Np on the periodic table. It has the atomic number 93 which means it has 93 protons and electrons in its atoms. It is named after the planet Neptune in the same way as Uranium is named after the planet Uranus.
History
Neptunium was discovered in the year of 1940 by two men named Edwin McMillan and Phillip H. Abelson at Berkeley Radiation Center of the University of California.[1] It was created by fusing Hydrogen nuclei called protons into the element Uranium.
Chemistry
Neptunium is a radioactive silvery-metallic element that is part of a group of elements called the Actinides, which it shares many similarities to. Its melting point is 637 degrees Celsius, its boiling point is 4000 degrees Celsius, and its atomic mass is 237.
It oxidizes very quickly with multiple different oxidation states. It also bonds with halides and even alkali metals.
Occurence
Since all isotopes of neptunium have half-lives that are many times shorter than the age of the Earth, any neptunium that was made when the earth was formed should have decayed by now. After only about 80 million years, the concentration of even the longest-lived isotope, 237Np, would have been reduced to less than one-trillionth (10−12) of its original amount.
Neptunium Media
The 4n + 1 decay chain of neptunium-237, commonly called the "neptunium series"
Dmitri Mendeleev's table of 1871, with an empty space at the position after uranium
Flowchart, showing the Purex process and the likely oxidation state of neptunium in the process solution
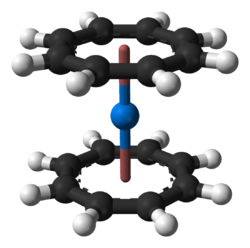
Ball-and-stick model of the neptunocene molecule, Np(C8H8)2], as found in the solid state.*X-ray crystallographic data from D. J. A. De Ridder, J. Rebizant, C. Apostolidis, B. Kanellakopulos and E. Dornberger (March 1996). "Bis(cyclooctatetraenyl)neptunium(IV)". Acta Cryst. C52 (3): 597-600. DOI:10.11
References
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).