Octet rule
The octet rule is a general rule of thumb that applies to most atoms. It states that every atom might want to have eight valence electrons in its outermost electron shell.
Background
According to the Bohr model, an atom consists of a nucleus of protons and neutrons orbited by a number of electrons. In an ordinary atom, the number of electrons equals the number of protons. If it has more or fewer electrons, it is an ion. In addition, the electrons orbit in electron shells. Each shell can only contain a certain number of electrons before new electrons must migrate to the next shell or "energy level." Starting with the third energy level, however, electrons sometimes move up a level even before the current one is full.
The rule
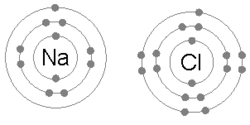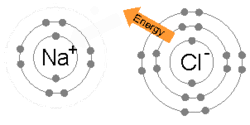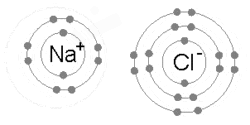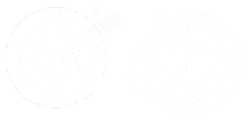
Every atom tries to have 8 electrons in its outermost (or valence) shell. This may require them to give up, share, or take electrons. As stated above, the number of electrons normally equals the number of protons. This means that some atoms are "happier" than others. For example, neon, as with other noble gases, naturally has 8 valence electrons. (10 total - 2 in the 1st energy level = 8 in the 2nd) Not surprisingly, neon hardly ever reacts with anything. (Neon lights require high voltages to work.) By contrast, the atoms in the Alkaline metals column, such as sodium, have only one valence electron, with a layer containing 8 electrons in the shell below. This means that sodium only has to give up one valence electron in order to complete the octet rule, and will do so if given the chance, hence why sodium is so chemically reactive.
Octet Rule Media
The bonding in carbon dioxide (CO2): all atoms are surrounded by 8 electrons, fulfilling the octet rule.
Newlands' law of octaves