Peroxy acid

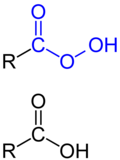
A peroxy acid,[1] also written peroxyacid or shortened to peracid,[2] is an acid where an acidic hydrogen atom is bonded to two consecutive oxygen atoms. They have a hydroperoxide functional group, –OOH.
Peroxy acids are weaker acids but stronger oxidizers than related oxoacids.
Production
Many oxoacids can be changed to peroxy acids in a chemical reaction with hydrogen peroxide. An example of this is the production of peroxymonosulfuric acid:[3]
- H
2SO
4 + H
2O
2 → H
2SO
5 + H
2O
Organic peracids can be made from carboxylic acids or their derivatives, like acid anhydrides or acyl chlorides.[4]
Uses
A major use for peroxy acids and their salts is the oxidation of alkenes into epoxides. If an organic peroxy acid is used, this is a name reaction called the Prilezhaev reaction.[2] Potassium peroxymonosulfate, sold commercially in a mix of salts called Oxone, is an inorganic peroxy acid salt used in a similar reaction.[5]
Peroxy Acid Media
References
- ↑ International Union of Pure and Applied Chemistry. "Peroxy acid". Compendium of Chemical Terminology Internet edition.
- ↑ 2.0 2.1 "Prilezhaev Reaction". Organic Chemistry Portal. Retrieved 2025-04-17.
- ↑ Colin F. McDonogh and Neil J. Sanders, "Manufacture of peroxidic compositions", US patent 5429812, issued 1995-07-04
- ↑ "Peroxy Acids and Esters". Organic Chemistry Portal. Retrieved 2025-04-17.
- ↑ Dan Yang, Yiu-Chung Yip, Guan-Sheng Jiao, and Man-Kin Wong (2002), "In Situ Catalytic Epoxidation of Olefins with Tetrahydrothiopyran-4-One and Oxone: trans-2-methyl-2,3-diphenyloxirane", Org. Synth., 78: 225
{{citation}}: CS1 maint: multiple names: authors list (link)