Ionic compound
In chemistry, an ionic compound is a compound made of ions. They have strong ionic bonds between particles. Lots of energy (heat) is required to break the bond apart, resulting in high melting and boiling points. The ions join because they have different charges. Compounds of metals and non-metals are usually ionic. They are made when two or more atoms come close together and an electron goes from one atom to the other atom. The electron does this because both atoms want a full outer shell, similar to those of the noble gases.
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | |
|---|---|---|---|---|---|---|---|---|
| Example Element | Na | Mg | Al | C | N | O | Cl | He |
| Charge | 1+ | 2+ | 3+ | See Note 1 | 3- | 2- | 1- | See Note 2 |
| Symbol of Ion | Na+ | Mg2+ | Al3+ | See Note 1 | N3- | O2- | Cl- | See Note 2 |
Note 1: carbon and silicon in Group 4 usually form covalent bonds by sharing electrons.
Note 2: the elements in Group 0 do not react with other elements to form ions
Physical properties
| Physical Properties | Ionic Compounds |
|---|---|
| State at room temperature | Solid |
| Electrical conductivity | As Solid: No
Liquid (melted): Yes Dissolved in solution: Yes (if soluble) |
| Boiling point and Melting Point | High |
| Solubility in water | Often high |
| Heat conductivity | Low |
Ionic Compound Media
Halite, the mineral form of sodium chloride, forms when salty water evaporates leaving the ions behind.
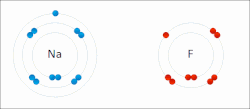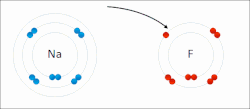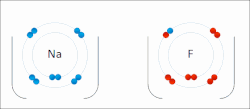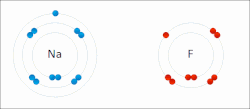
A schematic electron shell diagram of sodium and fluorine atoms undergoing a redox reaction to form sodium fluoride. Sodium loses its outer electron to give it a stable electron configuration, and this electron enters the fluorine atom exothermically. The oppositely charged ions – typically a great many of them – are then attracted to each other to form a solid.
The unit cell of the zinc blende structure