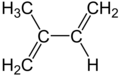
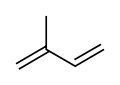
Isoprene
Isoprene is a common organic compound produced by living things. Its full chemical name is 2-methyl-1,3-butadiene, and its formula is CH2=C(CH3)−CH=CH2. Isoprene is an unsaturated hydrocarbon. It is produced by many plants and animals (including humans). Its polymers are the main component of natural rubber.[1][2][3]
| Isoprene | |
|---|---|
2-Methylbuta-1,3-diene | |
| Other names | 2-Methyl-1,3-butadiene Isoprene |
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | C16521 |
| ChEBI | CHEBI:35194 |
| SMILES | CC(=C)C=C |
| Properties | |
| Molecular formula | C5H8 |
| Molar mass | 68.12 g/mol |
| Density | 0.681 g/cm3 |
| Melting point |
−143.95 °C, 129 K, -227 °F |
| Boiling point | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Natural occurrences
Isoprene is produced and emitted by many species of trees (major producers are oaks, poplars, eucalyptus, and some legumes). Yearly production of isoprene emissions by vegetation is around 600 million metric tons, half from tropical broadleaf trees (that is, flowering plants) and the rest mainly from shrubs.[4]
This is about the same as methane emissions. It accounts for about one-third of all hydrocarbons released into the atmosphere. In deciduous forests, isoprene makes up about 80% of hydrocarbon emissions. Microscopic and macroscopic algae also produce isoprene, but much less than trees.[5]
Isoprene emission may help trees use to combat stress.[6] In particular, isoprene does protect against moderate heat stress (around 40 °C). It may also protect plants against large fluctuations in leaf temperature. Isoprene is built into cell membranes and helps membranes keep stable.
Isoprene produces the blue haze which gives the Blue Ridge Mountains their name.
Isoprene Media
Dimethylallyl diphosphate; Prenyl diphosphate; DMAPP; dimethylallyl-PPi; 3,3-dimethylallyl pyrophosphate
- PolyIsopreneCorrected.svg
Chemical structure of cis-polyisoprene, the main constituent of natural rubber
- Sterol synthesis.svg
Simplified version of the steroid synthesis pathway with the intermediates isopentenyl pyrophosphate (IPP), dimethylallyl pyrophosphate (DMAPP), geranyl pyrophosphate (GPP) and squalene shown. Some intermediates are omitted.
- EneIsoprene.svg
Production of isoprene from isobutene via ene reaction.
References
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ M. J. Loadman (2012-12-06). Analysis of Rubber and Rubber-like Polymers. Springer. p. 10. ISBN 9789401144353.
- ↑ Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P. I.; Geron, C. (2006). "Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature)". Atmospheric Chemistry and Physics. 6 (11): 3181–3210. Bibcode:2006ACP.....6.3181G. doi:10.5194/acp-6-3181-2006.
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).