Permanganate
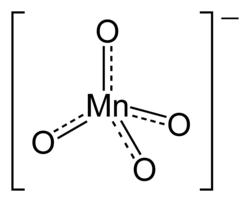
Permanganate is an ion that contains one manganese ion in an oxidation state of +7 and 4 oxide atoms. It is a strong oxidizing agent. Permanganates are purple-black. Permanganates stain because they are easily reduced to brown-black manganese dioxide.
| Permanganate | |
|---|---|
| |

| |
Permanganate | |
| Properties | |
| Molecular formula | MnO− 4 |
| Molar mass | 118.934 g mol-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
Examples
Permanganate Media
Absorption spectrum of an aqueous solution of potassium permanganate, showing a vibronic progression
Related pages
- Chromate, a similar ion
- Manganese
- Manganese dioxide


