Reduction (chemistry)
Reduction is a chemical reaction that involves the gaining of electrons by one of the atoms involved in the reaction between two chemicals. The term refers to the element that accepts electrons, as the oxidation state of the element that gains electrons is lowered.[1]
An example of a reduction is when iron reacts with oxygen, forming iron oxides such as those called rust. The iron is oxidized and the oxygen is reduced. This is called redox. A blast furnace reverses that reaction, using carbon monoxide as a reducing agent to reduce the iron.
Reduction (chemistry) Media
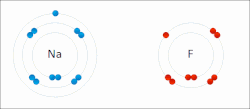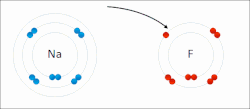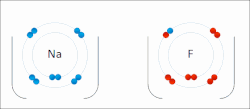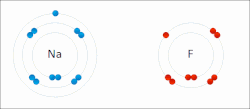
Sodium "gives" one outer electron to fluorine, bonding them to form sodium fluoride. The sodium atom is oxidized, and fluorine is reduced.
When a few drops of glycerol (mild reducing agent) are added to powdered potassium permanganate (strong oxidizing agent), a violent redox reaction accompanied by self-ignition starts.
The international pictogram for oxidizing chemicals
Illustration of a redox reaction
A redox reaction is the force behind an electrochemical cell like the Galvanic cell pictured. The battery is made out of a zinc electrode in a ZnSO4 solution connected with a wire and a porous disk to a copper electrode in a CuSO4 solution.
Oxides, such as iron(III) oxide or rust, which consists of hydrated iron(III) oxides Fe2O3·nH2O and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), form when oxygen combines with other elements.
Iron rusting in pyrite cubes
Enzymatic browning is an example of a redox reaction that takes place in most fruits and vegetables.
Blast furnaces of Třinec Iron and Steel Works, Czech Republic
Related pages
References
- ↑ Oxidation and Reduction, Khan Academy, retrieved 2017-05-22