Phosphite

Structural formula of phosphite ion
Phosphite (systematic name phosphonate) is an ion. Its chemical formula is HPO2−
3. It contains phosphorus in its +3 oxidation state. Chemical compounds containing the phosphite ion are called phosphites. Phosphites are the salts of phosphorous acid. They are poisonous. They are reducing agents. An example would be sodium phosphite.
Phosphite Media
The general structure of a phosphite ester showing the lone pairs on the P
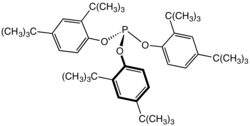
Tris(2,4-di-tert-butylphenyl)phosphite, a widely used stabilizer in polymers
BiPhePhos is representative diphosphite ligand used in homogeneous catalysis.