Selenium tetrachloride
Selenium tetrachloride, also selenious chloride, selenous chloride, or selenium(IV) chloride, is a chemical compound made of selenium and chlorine. Its chemical formula is SeCl
4. It contains selenium in its +4 oxidation state.
| Selenium tetrachloride | |
|---|---|
| |

| |
| IUPAC name | Selenium tetrachloride |
| Identifiers | |
| CAS number | |
| PubChem | |
| RTECS number | VS7875000 |
| SMILES | Cl[Se](Cl)(Cl)Cl |
| Properties | |
| Molecular formula | SeCl4 |
| Molar mass | 220.771 g/mol |
| Appearance | white to yellow crystals |
| Density | 2.6 g/cm³, solid |
| Melting point | |
| Solubility in water | decomposes in water |
| Structure | |
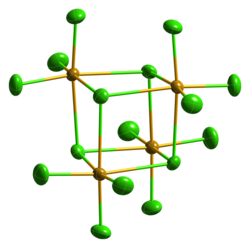
| Crystal structure | Monoclinic, mS80 |
| Space group | C12/c1, No. 15 |
| Molecular shape | Seesaw (gas phase) |
| Hazards | |
| EU classification | Toxic (T), Dangerous for the environment (N) |
| NFPA 704 |
|
| R-phrases | R23/25, R33, R50/53 |
| S-phrases | S20/21, S28, S45, S60, S61[2] |
| Flash point | non-flammable |
| Related compounds | |
| Other anions | Selenium tetrafluoride Selenium tetrabromide Selenium dioxide |
| Other cations | Dichlorine monoxide Sulfur tetrachloride Tellurium tetrachloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Properties
Selenium tetrachloride is a yellow or white solid. It evaporates easily. It reacts with water to make hydrochloric acid and selenous acid. It reacts with selenium dioxide to make selenium oxychloride, a mixture of selenium dioxide and selenium tetrachloride bonded together.
Preparation
It is made by heating a mixture of selenium and chlorine. The selenium tetrachloride escapes as a gas. This can be used to purify selenium. Impure selenium can be placed in the flask, reacted with chlorine. Only selenium tetrachloride escapes. This is reduced to selenium again, making pure selenium.
Uses
It is used to purify selenium. It is used to make other selenium compounds.
Related pages
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. p. 487. ISBN 0849305942. Retrieved 2008-07-02.
- ↑ "323527 Selenium tetrachloride". Sigma-Aldrich. Retrieved 2008-07-02.[dead link]
