Voltaic pile
The voltaic pile was the first ever battery. It was invented in 1800 by Alessandro Volta, an Italian physicist.
How the voltaic pile is made

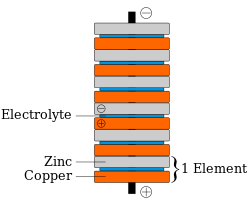
The picture to the right shows a voltaic pile: it's a pile, or "stack" of discs, made from 3 different materials. Some of the discs are made from copper, some from zinc. Other metals can be used; but it's important that there are two different kinds of metal. There are also discs made from leather, fabric, or cardboard: these are the green discs in the picture. They must be soaked, or "made wet", with something acidic.
Note how the discs follow a pattern: first a disc made from one of the metals, then a cardboard or leather disc, then a disc made from the other kind of metal. Then the same kinds of discs, in the same order, again and again. In the picture, you can see how one "set" of zinc disc, cardboard disc and copper disc has been "pushed" a little to the right. Such a "set" is called a cell: the voltaic pile in the picture has 6 cells. The two different metal discs in a cell will each have a voltage across it; about 1.1 volts. Stacking cells in a pile adds to the voltage of the cells, and from the discs at the top and bottom of the voltaic pile in the picture, there is a voltage of 6 × 1.1 volts = 6.6 volts.
How the voltaic pile works
The acid in the leather, fabric, or cardboard discs will try to dissolve some of the metal in the zinc discs. To be dissolved, a zinc atom must give away 2 of its electrons. The zinc atom then becomes an ion: Ions can "go where they want to" in the liquid acid. They go through the leather/cardboard disc, and meet the copper disc. The zinc ions are "missing" the two electrons they left behind, so when they get to the copper disc, they each "steal" two electrons to replace the missing ones. When they "steal" electrons, the zinc ions turn back from ions to "normal" zinc atoms. Saltwater can also be used in the cardboard, fabric, or leather, in a voltaic pile using copper and zinc plates.
The result is that the zinc disc ends up with a lot of electrons "left behind" by the zinc ions, and the copper disc ends up "missing" a lot of electrons "stolen" by the zinc ions. To use the electricity that comes from the pile, we must connect something that uses electricity to the two discs at the top and bottom of the pile. Doing so gives the electrons left behind in the zinc disc a "chance" to go to the copper disc. At the copper disc, the electrons "replace" those that were "stolen" from the copper by the zinc.
How Volta got the idea
Luigi Galvani was an Italian, working with anatomy. About 1786 he had seen something interesting: he had dissected (cut apart) a frog, and when he touched one of its legs with two tools made from different metals, it moved even though the frog had been killed before the dissection.
Galvani himself thought this had something to do with the leg, and not with the metal tools he used. Volta saw that the tools must be made from two different metals for the leg to move. Volta also found that he could make a little electricity just by making two different metals touch each other. He thought that the two different metals gave something he called contact tension, and the electricity came from that contact tension. Galvani and Volta talked a lot about this, but they never agreed.
Volta tried to make use of it all. He made experiments with (and tried out) rods of different kinds of metals, in wine carafes (a kind of bottle). This way, he found that the best metals to use, are silver and zinc. He could also make it better by using flat discs instead of rods of metal. This is because the discs have more surface area than the rod; this gives more "room" for the zinc ions to leave one disc and get in touch with the other.
Originally, Volta put an extra disc of each metal at the top and bottom of the stack. These were later proved to be unnecessary.
What happened after the invention
Before the voltaic pile was invented, people could make only static electricity. With the voltaic pile, they could make electricity that keeps flowing for some time. Along with this, it was an invention that could be easily made. With this kind of electricity, scientists could do a lot of "new" things. For instance, when new kinds of metal were discovered, the electricity from a voltaic pile could "sort" chemicals. This way, small but very pure bits of these "new" metals could be made.
Voltaic Pile Media
Schematic diagram of a copper–zinc voltaic pile. Each copper–zinc pair had a spacer in the middle, made of cardboard or felt soaked in salt water (the electrolyte). Volta's original piles contained an additional zinc disk at the bottom, and an additional copper disk at the top; these were later shown to be unnecessary.
A voltaic pile on display in the Tempio Voltiano (the Volta Temple) near Volta's home in Como, Italy
Voltaic pile, University History Museum of the University of Pavia.
Drawing of the voltaic pile in different configurations, from the letter sent from Alessandro Volta to Joseph Banks.