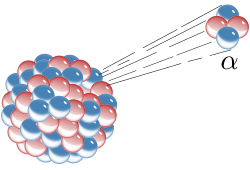
Alpha particle
An alpha particle is a radioactive particle that is made up of two protons and two neutrons. It is a helium nucleus: that is a helium atom without electrons. Radioactive elements give out alpha particles when they go through a kind of radioactive decay, known as alpha decay.
During alpha decay, the atomic nucleus releases an alpha particle. The nucleus will lose two protons and two neutrons when this happens. After the decay, the atom will change to another element, because the atom loses two protons. For example, when americium goes through alpha decay it changes into neptunium because neptunium is defined to have two protons less than americium. Alpha decay usually happens in heavy elements, those containing more neutrons and protons, such as uranium, thorium, plutonium, and radium.
Alpha particles cannot even go through a few centimetres of air. Alpha radiation cannot hurt humans when the alpha source is outside the human body, because human skin does not let the alpha particles go through, it cannot penetrate it. Alpha radiation can be very harmful if the source is inside the body, such as when people breathe dust or gas containing materials which decay by emitting alpha particles.
To look at americium again, the isotope americium-241 gives off alpha radiation. This radiation can be very harmful if it is inside someone's body because it can change the DNA in the cells. But it is not harmful when it is outside of the body because alpha radiation does not travel through the outside layers of the skin very well.
When scientists write equations about nuclear reactions they use the Greek letter Alpha. Ernest Rutherford started this when he discovered and named three kinds of ionizing radiation.
Alpha Particle Media
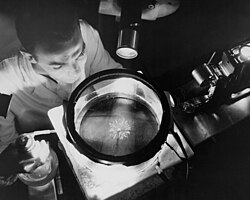
A physicist observes alpha particles from the decay of a polonium source in a cloud chamber
Alpha radiation detected in an isopropanol cloud chamber (after injection of an artificial source radon-220)
Example selection of radioactive nuclides with main emitted alpha particle energies plotted against their atomic number. Each nuclide has a distinct alpha spectrum.