Chloroform
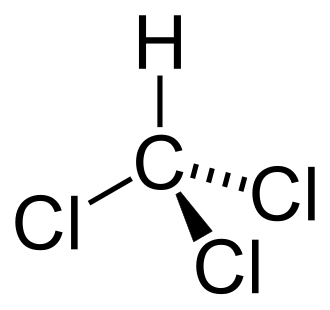
Chloroform (also called trichloromethane) is a chemical substance. It is an organic compound. Chloroform is one of the intermediate substances that occur in the production of Polytetrafluoroethylene, better known as Teflon. Chloroform is used as a solvent. It is a colourless liquid. In the 19th century, it was a widely used anaesthetic.[1]
History
The first who reported to have produced Chloroform was the French chemist Eugène Soubeiran, in 1831. Soubeiran took acetone and ethanol, and used bleach powder for the reaction.[2] The American physician Samuel Guthrie prepared gallons of the material and described its "deliciousness of flavor."[3] Independently, Justus von Liebig also described it.[4] Chloroform was named and chemically characterised in 1834 by Jean-Baptiste Dumas.[5] Starting in the 1850s factories made large amounts. Its use as an anesthetic declined in the early to mid-20th century as safer chemicals (like trichloroethylene and halothane) were more used.
Production
Chloroform is produced by mixing methane and chlorine at a temperature of 400 to 500°C. In this reaction, the result is a mix of chloromethane, methylene chloride, chloroform and carbon tetrachloride.
- [math]\displaystyle{ \mathrm{CH_4 + Cl_2 \longrightarrow CH_3Cl + HCl} }[/math]
- [math]\displaystyle{ \mathrm{CH_3Cl + Cl_2 \longrightarrow CH_2Cl_2 + HCl} }[/math]
- [math]\displaystyle{ \mathrm{CH_2Cl_2 + Cl_2 \longrightarrow CHCl_3 + HCl} }[/math]
- [math]\displaystyle{ \mathrm{CHCl_3 + Cl_2 \longrightarrow CCl_4 + HCl} }[/math]
The four compounds get then separated with a distillation.
In laboratories, chloroform can be produced by mixing sodium hypochlorite and acetone together. This is an exothermic reaction, meaning it makes a lot of heat.
Uses
The main uses of Chloroform are:
- as a solvent in factories and laboratories (especially chemical, biological and forensic)
- as an anaesthetic (from 1847 until the 1980s, no longer used)
- as a reagent to make other chemicals, especially refrigerants
Problems
Chloroform was used as an anaesthetic during childbirth and surgery, from about 1847. It replaced ether, which was used before. Chloroform is very poisonous, and can cause breathing problems, and problems with the heart. Death from chloroform can come from cardiac arrest. When Chloroform is stored for a longer time period, it can decay into Phosgene. Phosgene was a chemical weapon (poison gas) used during the First World War. Chloroform can cause birth defects and lead to miscarriages. It may cause cancer.
Chloroform Media
References
- ↑ "Chloroform - used, first, anesthetic, blood, body, Anesthetic Chloroform, Simpson Discovers Chloroforms Potency". discoveriesinmedicine.com. 2012. Retrieved 17 July 2012.
- ↑ Eugène Soubeiran (1831). "Annales de Chimie et de Physique". Ann. Chim. 48: 131. ISBN 9781012968083.
- ↑ Samuel Guthrie (1832). "New mode of preparing a spirituous solution of Chloric Ether". Am. J. Sci. And Arts. 21: 64.
- ↑ Justus Liebig (1832). "Ueber die Verbindungen, welche durch die Einwirkung des Chlors auf Alkohol, Aether, ölbildendes Gas und Essiggeist entstehen". Annalen der Pharmacie. 1 (2): 182–230. doi:10.1002/jlac.18320010203.
- ↑ Jean-Baptiste Dumas (1834). "Untersuchung über die Wirkung des Chlors auf den Alkohol". Annalen der Pharmacie. 107 (41): 650–656. Bibcode:1834AnP...107..650D. doi:10.1002/andp.18341074103.


