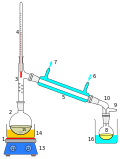
Distillation
1. heat source (a Bunsen burner here)
2. distilling flask (a round bottom flask)
3. distilling head
4. thermometer
5. condenser
6. cooling water in
7. cooling water out
8. receiving flask collecting dripping distillate
Distillation is a process where a mixture made of two or more liquids (called "components") with different boiling points can be separated from each other. The mixture is heated until one of the components boils (turns to a vapor). The vapor is then fed into a condenser, which cools the vapor and changes it back into a liquid that is called distillate. What remains in the original container is called the "residue". It is a common process in many industries.
Distillation is a physical separation process and not a chemical reaction. Some chemical processes are called "distillation" for historical reasons, like dry distillation or destructive distillation. These are a type of pyrolysis.
Types
Fractional distillation uses a larger distillation column with more than one output. It is used to separate mixtures of multiple liquids. An oil refinery uses fractional distillation to purify crude oil, separating several different liquids for different uses.
Air separation uses a cryogenic process to distill air. This produces pure nitrogen, oxygen, argon, and trace gases.
History
Distillation has been used for a long time. Iron Age people used distillation to make perfumes.[1] Pepole also distill alcohol and produce alcoholic drinks such as liquor.
Distillation can be done anywhere, whether it's in a house or a laboratory, but in most countries it is illegal to distill alcohol without a license. Illegally distilled alcoholic drinks are in some places called moonshine.[2]
Distillation is also the main way of desalination of water. In this case the salt is a solid that is in solution with the water. In alcohol distillation or petroleum distillation, the things to be separated from their solution are two or more distillation liquids.
Gallery
Distillation in a pharmacy, around 1900
Simple distillation of spirit, in East Timor
Distillation Media
- Zosimos distillation equipment.jpg
Distillation equipment used by the 3rd century alchemist Zosimos of Panopolis, from the Byzantine Greek manuscript Parisinus graces.
- DistillationbyRetort
Dimethyl sulfoxide usually boils at 189 °C. Under a vacuum, it distills off into the receiver at only 70 °C.
References
- ↑ Maria Rosaria Belgiorno (2020-05-25). "Ancient Distillation and Experimental Archaeology about the Prehistoric Apparatuses of Tepe Gawra". EXARC Journal.
- ↑ "Moonshine 'tempts new generation'". BBC News. 18 July 2010. https://www.bbc.co.uk/news/world-us-canada-10556048. Retrieved 2013-06-23.