Isomer

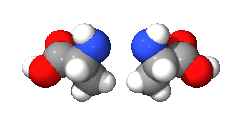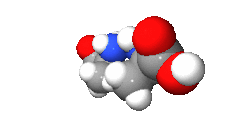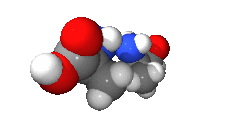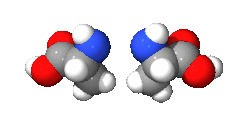
An isomer in chemistry is a chemical compound that has the same molecular formula as another, but it has a different arrangement of atoms in space. In other words, isomers are compounds with the same molecular formula but different structural formula. Different isomers have different chemical properties (that is, they may look, or smell, or taste different from each other).[1][2]
There are two main categories: stereoisomers and structural isomers. Structural isomers are isomers with the atoms making up the molecule joined together in different ways. Structural isomers can be classified further as chain isomers, position isomers, function group isomers, tautomers metamers and ring chain isomers.[3]
Stereoisomers have different positions of atoms in space and are much more subtle than structural isomers. See enantiomer for stereoisomers that are related to each other by a reflection: they are mirror images of each other that are non-superimposable.
Isomer Media
The two isomeric complexes, cisplatin and transplatin, are examples of square planar MX2Y2 molecules with M = Pt.
References
- ↑ "Isomer - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2021-04-09.
- ↑ "isomerism | Definition, types, & examples". Encyclopedia Britannica. Retrieved 2021-04-09.
- ↑ "A Brief Guide to Types of Isomerism in Organic Chemistry". Compound Interest. 2014-05-22. Retrieved 2021-04-09.
+{{{1}}}−{{{2}}}
+{{{1}}}−{{{2}}}