Nonmetal
Nonmetals or non-metals are chemical elements which do not have the properties of a metal.
They gain electrons when reacting with a metal. They are generally not lustrous and are bad conductors of heat and electricity. Some are gases including: hydrogen, helium, oxygen, nitrogen, fluorine, neon or radon and others. An example of a solid that is a nonmetal is sulfur. It is yellow and not shiny at all. An example of a liquid that is a nonmetal is bromine. It is red. A non-metal is a good insulator for heat and cold. Usually, gases or brittle solids are non-metals.
Elements on the periodic table can be classified as metal, semimetal, or non-metal. Five times more elements are metals than nonmetals. However, nonmetals are abundant and important. Two of the nonmetals—hydrogen and helium—make up over 99 percent of the observable Universe, and one—oxygen—makes up close to half of the Earth's crust, oceans and atmosphere. Living organisms are also composed almost entirely of nonmetals, and nonmetals form many more compounds than metals.
+{{{1}}}−{{{2}}}
+{{{1}}}−{{{2}}}
Nonmetal Media
While arsenic (here sealed in a container to prevent tarnishing) has a shiny appearance and is a reasonable conductor of heat and electricity, it is soft and brittle and its chemistry is predominately nonmetallic.
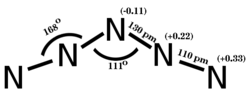
Molecular structure of pentazenium, a homopolyatomic cation of nitrogen with the formula N5+ and structure N−N−N−N−N.
A small (about 2 cm long) piece of rapidly melting argon ice
Selenium conducts electricity around 1,000 times better when light falls on it, a property used in light-sensing applications.
Cylinders containing argon gas for use in extinguishing fire without damaging computer server equipment
Bust of Dupasquier (1793–1848) in the Monument aux Grands Hommes de la Martinière in Lyon, France.