Phosphorus(V) oxide
Phosphorus(V) oxide, also known as phosphorus pentoxide, is a chemical compound. Its chemical formula is P4O10. It contains phosphorus and oxide ions. It contains phosphorus in its +5 oxidation state.
Properties
Phosphorus(V) oxide is a colourless solid. It has a strong odor. It dissolves in water to produce phosphoric acid. It can corrode metals. It is irritating to the skin. It can make bad burns.
Preparation
It is made by burning phosphorus into high amounts of air.
Uses
It is used to dry things out because it absorbs water. It dries things out from acids like nitric acid to organic compounds like carboxylic acids.
Safety
Phosphorus(V) oxide is corrosive. Its vapors are toxic and burn the eyes and skin. It also irritates skin and eyes.
Phosphorus(V) Oxide Media
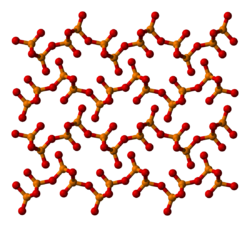
Ball-and-stick model of part of the crystal structure of o′-(P2O5)∞, the thermodynamically most stable form of phosphorus pentoxide. The structure consists of stacked infinite sheets of six-membered rings*X-ray crystallographic data from D. Stachel, I. Svoboda and H. Fuess (June 1995). "Phosphorus Pentoxide at 233 K". Acta Cryst. C51 (6): 1049-1050. DOI:
Ball-and-stick model of part of the crystal structure of o′-(P2O5)∞, the thermodynamically most stable form of phosphorus pentoxide. The structure consists of stacked infinite sheets of six-membered rings*X-ray crystallographic data from D. Stachel, I. Svoboda and H. Fuess (June 1995). "Phosphorus Pentoxide at 233 K". Acta Cryst. C51 (6): 1049-1050. DOI: