Thermosetting polymer
A thermosetting polymer, also known as a thermoset, is polymer material that irreversibly cures. The curing may be done by:
• heat (generally above 200 °C (392 °F))
• a chemical reaction (two-part epoxy, for example)
• irradiation such as electron beam processing
Thermoset materials are usually liquid or malleable before curing, so they can be molded into their final form. Others are used as adhesives. Others are solids. Some solid thermosetting polymers are used as molding compound in semiconductors and integrated circuits (IC). Once hardened a thermoset resin cannot be reheated and melted back to a liquid form.
Process
The curing process transforms the resin into a plastic or rubber by a cross-linking process. Energy and/or catalysts are added that cause the molecular chains to react at chemically active sites (unsaturated or epoxy sites, for example), linking into a rigid, 3-D structure. The cross-linking forms a molecule with a larger molecular weight, resulting in a material with a higher melting point. During the reaction, the polymer's molecular weight increases to the point that its melting point is higher than the surrounding ambient temperature. So, the material forms into a solid material.
Uncontrolled reheating of the material results in reaching the decomposition temperature before the melting point is reached. So, a thermoset material cannot be melted and re-shaped after it is cured. This implies that thermosets cannot be recycled, except as filler material.[1]
Properties
Thermoset materials are generally stronger than thermoplastic materials due to this three dimensional network of bonds (cross-linking). Thermoset materials are also better suited to high-temperature applications up to the decomposition temperature. However, they are more brittle. Many thermosetting polymers are difficult to recycle.
Thermosetting Polymer Media
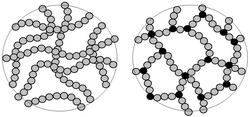
Left: individual linear polymer chainsRight: Polymer chains which have been cross linked to give a rigid 3D thermoset polymer
Related pages
References
- ↑ The Open University (UK), 2000. T838 Design and Manufacture with Polymers: Introduction to Polymers, page 9. Milton Keynes: The Open University