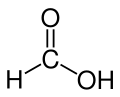
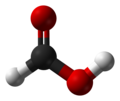
Formic acid
Formic acid, or methanoic acid, is the simplest carboxylic acid. The chemical formula is HCOOH. Many animals use for defence. The word "formic" comes from the Latin word for ant, formica, referring to its early isolation by the distillation of ant bodies, and the trivial name in some languages means "ant-acid", such as Dutch mierenzuur, Danish myresyre, Faroese meyrusýra, Français acide formique and German Ameisensäure. Esters, salts, and the anions derived from formic acid are called formates.
| Formic acid | |
|---|---|

| |
Formic acid[1] | |
Methanoic acid[1] | |
| Other names | Carbonous acid; Formylic acid; Hydrogen carboxylic acid; Hydroxy(oxo)methane; Metacarbonoic acid; Oxocarbinic acid; Oxomethanol |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 200-579-1 |
| DrugBank | DB01942 |
| KEGG | C00058 |
| ChEBI | CHEBI:30751 |
| RTECS number | LQ4900000 |
| SMILES | O=CO |
| Properties | |
| Molecular formula | CH2O2 |
| Molar mass | 46.01 g mol-1 |
| Appearance | Colorless fuming liquid |
| Odor | Pungent, penetrating |
| Density | 1.220 g/mL |
| Melting point |
8.4 °C, 282 K, 47 °F |
| Boiling point | |
| Solubility in water | Miscible |
| Solubility | Miscible with ether, acetone, ethyl acetate, glycerol, methanol, ethanol Partially soluble in benzene, toluene, xylenes |
| log P | −0.54 |
| Vapor pressure | 35 mmHg (20 °C)[2] |
| Acidity (pKa) | 3.77[3] |
| -19.90·10−6 cm3/mol | |
| Refractive index (nD) | 1.3714 (20 °C) |
| Viscosity | 1.57 cP at 268 °C |
| Structure | |
| Molecular shape | Planar |
| Dipole moment | 1.41 D (gas) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−425.0 kJ/mol |
| Std enthalpy of combustion ΔcH |
−254.6 kJ/mol |
| Standard molar entropy S |
131.8 J/mol K |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Corrosive; irritant; sensitizer |
| NFPA 704 |
|
| R-phrases | Template:R10 R35 |
| S-phrases | (S1/2) S23 S26 S45 |
| Explosive limits | 14–34%[source?] 18%–57% (90% solution)[2] |
| U.S. Permissible exposure limit (PEL) |
TWA 5 ppm (9 mg/m3)[2] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
In the 15th century, many alchemists reported that ants use an acidic fluid for defense. English naturalist John Ray was the first to get formic acid, by distilling ants, in 1671.
In nature, it is found in most ants.[4] The wood ants from the genus Formica can spray formic acid on their preys or to defend the nest. It is also known from the trichomes of stinging nettle (Urtica dioica). Formic acid is a naturally occurring component of the atmosphere due primarily to forest emissions.[5]
Formic Acid Media
References
- ↑ 1.0 1.1 Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. p. 745. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 NIOSH Pocket Guide to Chemical Hazards. "#0296". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Brown, H. C. et al., in Braude, E. A. and Nachod, F. C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
- ↑ Hoffman, Donald R. "Ant venoms" Current Opinion in Allergy and Clinical Immunology 2010, vol. 10, pages 342–346.
- ↑ Sanhueza, E., & Andreae, M. O. (1991). "Emission of formic and acetic acids from tropical savanna soils". Geophysical Research Letters. 18 (9): 1707–1710. Bibcode:1991GeoRL..18.1707S. doi:10.1029/91GL01565.
{{cite journal}}: CS1 maint: uses authors parameter (link)