Mass
The mass of an object is a measure of how much of it there is.[1] For example, A mountain has more mass than a rock. Mass is different from weight. Weight is the product of mass times gravitational acceleration.
You can measure the mass of an object by using a force on it. The more mass it has, the less it will change how fast it moves. This is called inertial mass because it measures the inertia.[2]
An object with a lot of mass, like a planet attracts objects with less mass and keeps them from floating away from the planet. This is called gravity. a When measuring the force of gravity on an object, you can find the object’s gravitational mass. Tests of inertial and gravitational mass show that they are the same or almost the same.[2]
Units of mass
The unit of mass in the International System of Units is the kilogram, which is represented by the symbol 'kg'. Fractions and multiples of this basic unit include the gram (one thousandth of a kg, symbol 'g') and the tonne (one thousand kg), amongst many others.
In some fields or applications, it is convenient to use different units to simplify the discussions or writings. For instance,
- Atomic physicists deal with the tiny masses of individual atoms and measure them in atomic mass units.
- Jewelers normally work with small jewels and precious stones where masses are traditionally measured in carats, which correspond to 200 mg or 0.2 g.
- The masses of stars are very large and are sometimes expressed in units of solar masses.
Traditional units are still in encountered in some countries: imperial units such as the ounce or the pound were in widespread use within the British Empire. Some of them are still popular in the United States, which also uses units like the short ton (2,000 pounds, 907 kg) and the long ton (2,240 pounds, 1010 kg), not to be confused with the metric ton (1,000 kg).
Conservation of mass and relativity
Mass Media
The kilogram is one of the seven SI base units.
Mass and weight of a given object on Earth and Mars. Weight varies due to different amount of gravitational acceleration whereas mass stays the same.
Astronaut David Scott performs the feather and hammer drop experiment on the Moon.
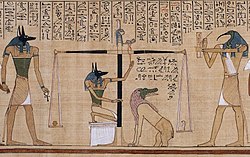
Depiction of early balance scales in the Papyrus of Hunefer (dated to the 19th dynasty, c. 1285 BCE). The scene shows Anubis weighing the heart of Hunefer.
A cannon on top of a very high mountain shoots a cannonball horizontally. If the speed is low, the cannonball quickly falls back to Earth (A, B). At intermediate speeds, it will revolve around Earth along an elliptical orbit (C, D). Beyond the escape velocity, it will leave the Earth without returning (E).
Mass is an intrinsic property of the object: it does not depend on its volume, or position in space, for instance. For a long time (at least since the works of Antoine Lavoisier in the second half of the eighteen century), it has been known that the sum of the masses of objects that interact or of the chemicals that react remain conserved throughout these processes. This remains an excellent approximation for everyday life and even most laboratory work.
However, Einstein has shown through his special theory of relativity that the mass m of an object moving at speed v with respect to an observer must be higher than the mass of the same object observed at rest m0 with respect to the observer. The applicable formula is
[math]\displaystyle{ m=\frac{m_0}{\sqrt{1 - (v^2/c^2)}} }[/math]
where c stands for the speed of light. This change in mass is only important when the speed of the object with respect to the observer becomes a large fraction of c.
The Quantum Concept of Mass
For further reference,see the Gluon field and Higgs boson
In atomic nuclei, example in protons and neutrons,the residual mass comes from the binding kinetic and potential energy of the quarks and gluon field. An analogy to go along with is to think of the 3 quarks as balls with the gluons as a spring connecting the quarks. This mass accounts for 99% of the mass of these baryons with the remaining 1% coming from the individual quarks which comes from quantum interactions with the Higgs field.
References
- ↑ Tsokos, K. A. (2005). Physics for the IB Diploma. Cambridge, United Kingdom: Cambridge University Press. p. 63. ISBN 9780521604055.
- ↑ 2.0 2.1 Knight, Randall Dewey (2003). Physics for scientists and engineers with modern physics : a strategic approach. San Francisco: Pearson/Addison-Wesley. pp. 349. ISBN 0-321-24329-3. OCLC 54427199.
Related pages
- Mass versus weight
- Center of mass
- Gravity
- Density
- Body mass index
- Momentum
- Advanced topics: