Neodymium
Neodymium is a chemical element that has the symbol Nd on the periodic table. It has the atomic number 60 which means it has 60 protons in an atom. Neodymium can be combined with other elements like iron and boron to create the very strong neodymium magnet, these magnets can lift up to 1000 times their own weight. Neodymium is a metal that forms a coating of oxide around itself when it is placed in air. It is usually used as neodymium magnets for hard disk drives.
Properties
Though neodymium is called a rare earth metal, it is quite common, as common as nickel, cobalt or copper. Like every metal, neodymium is very shiny. It is a solid that melts at 1,024 degrees Celsius, or 1,875 degrees Fahrenheit. Neodymium is in a group of other elements called lanthanides, which are like each other in how they behave. When neodymium is dropped into water, it slowly changes into neodymium hydroxide, if the water or the neodymium itself is hot enough, the change is much faster, and the neodymium may explode. In the air, if the neodymium is hot enough, it can catch fire. Like every other metal, neodymium usually wants to get rid of its electrons, so the compounds it forms are usually with elements that want electrons, as oxygen does.
Uses
The neodymium magnets used to make hard disk drives do not weaken over time: they are permanent magnets. They can also be used in microphones or speakers. Electric guitars may use these magnets inside their pickups.
Estimates of neodymium that can be mined
"China is the largest neodymium supplier and exporter in the world—dominating the neodymium industrial chain. The global neodymium reserve is estimated to be 11.6 million tons ... — a 13.6 million tons of neodymium oxide equivalent", according to media.[1]
Neodymium Media
Carl Auer von Welsbach (1858–1929), who discovered neodymium in 1885
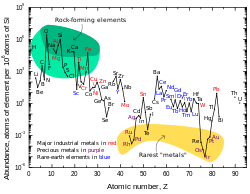
Neodymium is a fairly common element in the Earth's crust for being a rare-earth metal. Most rare-earth metals are less abundant.
A neodymium glass light bulb, with the base and inner coating removed, under two different types of light: fluorescent on the left, and incandescent on the right.
Sources
- ↑ https://www.sciencedirect.com/science/article/abs/pii/S092134492100361X. Retrieved 2024-06-08