Sulfonic acid
A sulfonic acid is a chemical compound related to sulfuric acid, where one of the pairs of oxygen and hydrogen atoms (called a hydroxy group, –OH) in sulfuric acid is replaced with a carbon atom. Because of the chemical bond between sulfur and carbon, sulfonic acids are a type of organosulfur compound.[1] Sulfonic acids are ingredients in detergents (alkylbenzene sulfonate), medications (sulfa drugs), and some dyes.
The parent compound, which has hydrogen instead of carbon, would also be called "sulfonic acid" (HSO
2OH). but research has shown that it is a tautomer of sulfurous acid (H
2SO
3), meaning that the two chemicals change into each other so easily that they can never be separated. Pure sulfurous acid has never been produced, as it disproportionates to sulfur dioxide and water.
Sulfonic Acid Media
Ball-and-stick model of methanesulfonic acid.
Acamprosate is a sulfonic acid-containing drug use to treat alcohol use disorder.
Sulfonates are the basis of most ion exchange resins used in water softening.
PFOS, a surfactant and a controversial pollutant.
p-Toluenesulfonic acid, a widely used reagent in organic synthesis.
Nafion, a polymeric sulfonic acid useful in fuel cells.
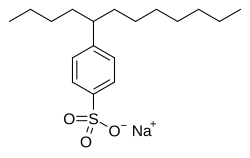
Sodium dodecylbenzenesulfonate, an alkylbenzenesulfonate surfactant used in laundry detergents.
References
- ↑ International Union of Pure and Applied Chemistry. "Sulfonic acids". Compendium of Chemical Terminology Internet edition.