Prussian blue
Prussian blue, also known as Berlin blue, is a dark blue color that is artificially made.
| Prussian blue | |
|---|---|
| IUPAC name | Iron(II,III) hexacyanoferrate(II,III) |
| Other names |
|
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 237-875-5 |
| ChEBI | CHEBI:30069 |
| SMILES | [Fe+3].[Fe+3].[Fe+3].[Fe+3].N#C[Fe-4](C#N)(C#N)(C#N)(C#N)C#N.N#C[Fe-4](C#N)(C#N)(C#N)(C#N)C#N.N#C[Fe-4](C#N)(C#N)(C#N)(C#N)C#N |
| Gmelin Reference | 1093743 |
| Properties | |
| Molecular formula | C18Fe7N18 |
| Molar mass | 859.23 g mol-1 |
| Appearance | Blue opaque crystals |
| Pharmacology | |
| ATC code | |
| Oral | |
| Related compounds | |
| Other cations | Potassium ferrocyanide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Prussian blue | |
|---|---|
| Hex triplet | #003153 |
| sRGBB (r, g, b) | (0, 49, 83) |
| HSV (h, s, v) | (205°, 100%, 32%) |
| Source | [1] |
| ISCC–NBS descriptor | Dark blue |
| B: Normalized to [0–255] (byte) | |
It is one of the first pigments made synthetically. It was accidentally found in 1704 by two chemists in Berlin.[1]
The dark blue uniforms of the Prussian army were dyed this color.[2]
Color
A color can be shown by hex triplet is a six-digit, three-byte hexadecimal number used in HTML, CSS, and other computing applications. The hex triplet to represent Prussian blue is 003153.
When using the RGB color model where red, green, and blue light are added together, Prussian blue has the RGB code of 0, 49, 83
Use as a medicine
Prussian blue is a medicine that is sometimes given by doctors to help remove certain radioactive materials from people’s bodies. It has been used this way since the 1960s.[3]
Potential use in computer storage
Prussian blue is a chemical compound. One of the chemical elements in this compound is iron.[2]
Researchers have experimented replacing some of the iron atoms in Prussian blue with cobalt. When the Prussian blue compound is lit with a red light at -150 C, the compound shifts from being non-magnetic (off) to magnetic (on). The magnetic shift does not change back except if deliberately reversed (or undone) with heat.[2]
This magnetism is due to the transfer of an electron from the cobalt to the iron, with light providing the energy, while the electron moves back when heat is applied, the researchers said.[2]
This magnetic property means the compound can be used in computer storage. Because the compound can be turned "on" and "off" in a controlled way, it can remember binary information. Binary information is used for computer storage.[2]
This way of using the compound is still being developed.[2]
It is also used as a machinists dye to check wear patterns.
Tones of Prussian blue color comparison chart
| Name | Color | HEX Code | Red | Green | Blue | Hue | Sat | Lum | Source |
|---|---|---|---|---|---|---|---|---|---|
| Dark Slate Blue | #483D8B | 72 | 61 | 139 | 248° | 39% | 39% | (web color) | |
| Persian Indigo | #32127A | 50 | 18 | 122 | 258° | 74% | 27% | (Regimental) (PerBang.dk) (Maerz & Paul) | |
| Dark Imperial Blue | #00416A | 0 | 65 | 106 | 203° | 100% | 21% | Imperial Blue (ISCC-NBS) | |
| Midnight Blue | #191970 | 25 | 25 | 112 | 240° | 64% | 27% | (web color) | |
| Dark Midnight Blue | #003366 | 0 | 51 | 102 | 210° | 100% | 20% | (Midnight Blue (Crayola)) | |
| Prussian Blue | #003153 | 0 | 49 | 83 | 205° | 100% | 16% | (Berlin Blue) (PerBang.dk) (Maerz & Paul) | |
| Dark Indigo | #310062 | 49 | 0 | 98 | 270° | 100% | 19% | (PerBang.dk) | |
| Gulf Blue | #051657 | 9 | 22 | 87 | 228° | 89% | 18% | (Xona.com color list) |
Prussian Blue Media
Ferricyanide ion, used to make Turnbull's blue
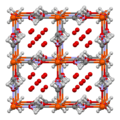
Fe coordination spheres in idealized Prussian blue
The unit cell of Prussian blue, with all sites occupied. Actually, one fourth of the Fe(CN)
6 groups shown will be missing, at random, giving on average only 18 cyanide ions (rather than the 24 shown) and three ferrous iron atoms.Simulated powder x-ray diffraction profile for Prussian blue crystal, crystallographic direction annotated. Image generated using CrystalMaker software.
The unit cell of Prussian blue determined by neutron diffraction, with crystallographically disordered water molecules both in cyanide ion positions and in the void space of the framework. Again, one fourth of the Fe(CN)
6 groups shown will be missing. This illustration superimposes both possibilities at each site — water molecules or cyanide ions.The clock faces of the Great Clock of Westminster, restored to their original 1859 color scheme of Prussian blue and gold
Cyclic voltammograms of Prussian Blue electrode in solution of different alkali cations
Related pages
References
- ↑ Boddy-Evans, Marion. "Artist's Pigments: The Accidental Discovery of Prussian Blue". Painting. About.com (New York Times). Archived from the original on 2008-10-05. Retrieved 2008-10-16.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Ancient dye could brighten computer storage. Radio Canada: CBCNews.ca. January 18, 2007. http://www.cbc.ca/technology/story/2007/01/18/prussian-blue.html. Retrieved 2008-10-16.
- ↑ "Radiation Emergencies > Emergency Instructions > Fact sheet: Prussian blue". Emergency Preparedness and Response. Centers for Disease Control and Prevention: US Government, Department of Health and Human Services. 2005. Archived from the original on 2013-10-20. Retrieved 2008-10-24.