Nucleotide

A nucleotide is an organic molecule. Nucleotides are the building blocks of the nucleic acids RNA and DNA. These two types of nucleic acid are essential biomolecules in all forms of life on Earth.
A nucleotide is composed of a nucleobase (nitrogenous base), a five-carbon sugar (either ribose or 2-deoxyribose), and one phosphate group.[1][2] Nucleotides contain either a purine or a pyrimidine base. Ribonucleotides are nucleotides in which the sugar is ribose. Deoxyribonucleotides are nucleotides in which the sugar is deoxyribose.
In DNA, the purine bases are adenine and guanine, and the pyrimidines are thymine and cytosine. RNA uses uracil in place of thymine. Adenine always pairs with thymine by 2 hydrogen bonds, while guanine pairs with cytosine through 3 hydrogen bonds, each due to their unique structures.
Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy for the many cellular functions that need it. Examples are: amino acid, protein and cell membrane synthesis, moving the cell and cell parts (both internally and intercellularly), cell division, and so on.[2] In addition, nucleotides work in cell signaling, and they are in important cofactors of enzymatic reactions (e.g. coenzyme A, FAD, FMN, NAD, and NADP+).
In experimental biochemistry, nucleotides can be labeled using radionuclides to make radionucleotides.
Nucleotide Media
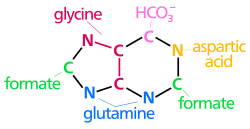
The synthesis of UMP.* enzymes* coenzymes* substrate names* metal ions* inorganic molecules
References
- ↑ Coghill, Anne M.; Garson, Lorrin R., ed. (2006). The ACS style guide: effective communication of scientific information (3rd ed.). Washington D.C.: American Chemical Society. p. 244. ISBN 978-0-8412-3999-9.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ↑ 2.0 2.1 Alberts B et al 2002. Molecular biology of the cell. 4th ed, Garland Science, 120–121. ISBN 0-8153-3218-1