Photosynthesis

Photosynthesis is a process in which green plants making their own food from sunlight through the help of leaves to produce sugar. It is the process by which these organisms convert light energy into chemical energy, which is used to produce food.
Origin
Photosynthesis is not an accident. It happens because its building blocks occur in space, and gets included when planets form. We have no idea as to how common or rare this process is. We do know that higher elements are formed in supernovae, and that is the origin of all elements higher than helium.[1] The higher elements found in Halley's Comet have been analysed.[2][3]
Before photosynthesis, the Earth's atmosphere had almost no oxygen. Even without oxygen, some simple life-forms could have existed. But the key event for life as we know it was the Great Oxygenation Event.[4]
Ways it is done
Photosynthesis can happen in different ways, but there are some parts that are common.[5]
- 6 CO2(g) + 6 H2O + photons → C6H12O6(aq) + 6 O2(g)
- carbon dioxide + water + light energy → glucose + oxygen
- Carbon dioxide enters the leaf through the stomata by diffusion from the atmosphere.
- Water is absorbed from the soil by root hair cells, which have an increased surface area adapted for their uptake of water.
Photosynthesis occurs in the chloroplasts in leaves (or other green tissues). They contain chlorophyll, the green pigment that absorbs light energy. In leaves, palisade cells have chloroplasts to capture light.
Oxygen is produced as a result of photosynthesis and released into the atmosphere through respiration. All the oxygen in the atmosphere has its origin in plants (including those microorganisms which do photosynthesis).
Glucose is used in respiration (to release energy in cells). It is stored in the form of starch (which is converted back to glucose for respiration in the dark). Glucose can also be converted into other compounds for growth and reproduction e.g. cellulose, nectar, fructose, amino acids and fats.
Process
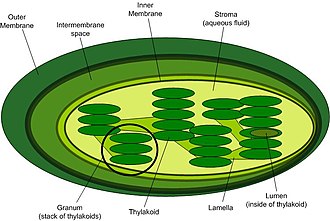


Photosynthesis has two main sets of reactions, known as phases. Light-dependent reactions which need light to do work; and light-independent reactions which do not need light to do work.
Light-dependent phase
Light energy from the sun is used to split water molecules (photolysis). The sunlight hits chloroplasts in the plant. This causes an enzyme to split the water. Water, when split, gives oxygen, hydrogen, and electrons.[7]
Hydrogen, along with electrons energized by light, converts NADP into NADPH which is then used in the light-independent reactions. Oxygen gas diffuses out of the plant as a waste product of photosynthesis, and ATP is synthesized from ADP and inorganic phosphate. This all happens in the grana of chloroplasts.
Light-independent reaction
During this reaction, sugars are built up using carbon dioxide and the products of the light-dependent reactions (ATP and NADPH) and various other chemicals found in the plant in the Calvin Cycle. Therefore, the light-independent reaction cannot happen without the light-dependent reaction. Carbon dioxide diffuses into the plant and along with chemicals in the chloroplast, ATP, and NADPH, glucose is made and finally, transported around the plant by translocation.
Factors affecting photosynthesis
There are three main factors affecting photosynthesis:
Light intensity
If there is little light shining on a plant, the light-dependent reactions will not work efficiently. This means that photolysis (breakdown of water by light) will not happen quickly, and therefore little NADPH and ATP will be made. This shortage of NADPH and ATP will lead to the light-independent reactions not working as NADPH and ATP are needed for the light-independent reactions to work.
The light intensity required is easily investigated in an aquatic plant such as pondweed. The oxygen bubbles given off can be counted or the volume measured. By changing the distance between light and plant, the light intensity is made to vary. Change in light intensity will affect the change in rate of photosynthesis. Artificial lighting can be used in the dark to maximise the photosynthetic rate.
Carbon dioxide levels
Carbon dioxide is used in the light-independent reactions. It combines with NADPH and ATP and various other chemicals to form glucose. Therefore, if there is not enough carbon dioxide, then there will be a build-up of NADPH and ATP and not enough glucose will be formed.
Temperature
There are many enzymes working in photosynthetic reactions – such as the enzyme in photolysis. All enzymes work best at their optimum temperature. All light-dependent and light-independent reactions will occur normally at average or optimum temperatures. Tropical plants have a higher temperature optimum than the plants adapted to temperate climates.
When the temperatures are too low, there is little kinetic energy, so the reaction rate decreases. If the temperatures are too high, the enzymes become denatured and the catalysis of photosynthesis reaction stops.
Greenhouses must keep an optimum temperature for normal functioning of plants.
Early evolution
The first photosynthetic organisms probably evolved early in the history of life. They may have used reducing agents such as hydrogen or hydrogen sulfide as sources of electrons, rather than water.[8] Cyanobacteria appeared later, and the excess oxygen they produced caused the oxygen catastrophe.[9][10] After this, the evolution of complex life was possible.
Differences
The photosystems of green sulfur bacteria and those of cyanobacteria, algae, and higher plants are not the same. That shows photosynthesis occurred not once, but several times over.[11]
Effectiveness
Today, the average rate of energy capture by photosynthesis globally is about 130 terawatts.[12][13] This is about six times larger than the current power used by human civilization.[14] Photosynthetic organisms also convert about 100–115 thousand million metric tonnes of carbon into biomass per year.[15][16]
Photosynthesis Media
Composite image showing the global distribution of photosynthesis, including both oceanic phytoplankton and terrestrial vegetation. Dark red and blue-green indicate regions of high photosynthetic activity in the ocean and on land, respectively.
Chloroplast ultrastructure:Template:Ordered list*
Overview of the Calvin cycle and carbon fixation
Overview of C4 carbon fixation. (This image mistakenly shows lactic acid instead of pyruvate, and all the species ending in "-ate" are shown as unionized acids, such as malic acid and so on).
Plant cells with visible chloroplasts (from a moss, Plagiomnium affine)
Portrait of Jan Baptist van Helmont by Mary Beale, c. 1674
Related pages
References
- ↑ Bryant D.A. & Frigaard N.U. 2006. Prokaryotic photosynthesis and phototrophy illuminated. Trends in Microbiology 14, p488–496. PMID 16997562.
- ↑ Brandt, John C. McGraw−Hill AccessScience: Halley's Comet. McGraw-Hill.
- ↑ Oro J. Historical understandings of life's beginnings. In J. William Schopf (editor). 2002. Life's origin. University of California Press. ISBN 0-520-23391-3
- ↑ Cloud, Preston E. 1968. Atmospheric and hydrospheric evolution on the primitive Earth. Science 160 (3829): 729–736. PMID 5646415 [1]
- ↑ Walker, J.C.G. (1980). The oxygen cycle in the natural environment and the biogeochemical cycles. Berlin: Springer-Verlag.
- ↑ Understanding global change https://ugc.berkeley.edu/background-content/respiration. Retrieved November 30, 2024.
{{cite web}}: Missing or empty|title=(help) - ↑ Dolai U. 2017. Chemical scheme of water-splitting process during photosynthesis by the way of experimental analysis. IOSR Journal of Pharmacy and Biological Sciences 12(6): 65-67. doi:10.9790/3008-1206026567. ISSN 2319-767
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Awramik S.M. 1992. The oldest records of photosynthesis. Photosynth Res 33 (2):75-89. doi:10.1007/BF00039172. PMID: 24408570 [2]
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Steger U; et al. (2005). Sustainable development and innovation in the energy sector. Berlin: Springer. p. 32. ISBN 3-540-23103-X.
The average global rate of photosynthesis is 130 TW (1 TW = 1 terawatt = 1012 watt).
- ↑ "World consumption of primary energy by energy type and selected country groups, 1980–2004" (XLS). Energy Information Administration. 2006. Retrieved 2007-01-20.
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ "Photosynthesis". McGraw-Hill Encyclopedia of Science & Technology. Vol. 13. New York: McGraw-Hill. 2007. ISBN 978-0-07-144143-8.