Nihonium
Nihonium (ニホニウム) is a chemical element. It is also named eka-thallium. It has the symbol Nh. It has the atomic number 113. It is a transuranium element. The name "nihonium" comes from the name of Japan in Japanese, 日本 (nihon).
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /nɪˈhoʊniəm/ | |||||||||||||||||||||||||||||||||||||||||||||||
| Mass number | 286 (most stable isotope) | |||||||||||||||||||||||||||||||||||||||||||||||
| Nihonium in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 113 | |||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 13 (boron group) | |||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | |||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||||||||||||||
| Element category | unknown chemical properties, but probably a post-transition metal | |||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d10 7s2 7p1 (predicted)[1] | |||||||||||||||||||||||||||||||||||||||||||||||
Electrons per shell | 2, 8, 18, 32, 32, 18, 3 (predicted) | |||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | Nh: Unknown phase (predicted)[1][2][3] | |||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 700 K (430 °C, 810 °F) (predicted)[1] | |||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1430 K (1130 °C, 2070 °F) (predicted)[1][4] | |||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 16 g/cm3 (predicted)[4] | |||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.61 kJ/mol (extrapolated)[3] | |||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 130 kJ/mol (predicted)[2][4] | |||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | (−1), (+1), (+3), (+5) (predicted)[1][4][5] | |||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 170 pm (predicted)[1] | |||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 172–180 pm (extrapolated)[3] | |||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | Nh: Synthetic | |||||||||||||||||||||||||||||||||||||||||||||||
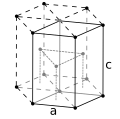
| Crystal structure | hexagonal close-packed (hcp) (extrapolated)[6] | |||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 54084-70-7 | |||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | After Japan (Nihon in Japanese) | |||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Riken (Japan, first undisputed claim 2004) JINR (Russia) and Livermore (US, first announcement 2003) | |||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes of nihonium | ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
Nihonium does not exist in nature, and can only be made artificially. It is made from the alpha decay of moscovium.
There are no known uses for nihonium. What nihonium looks like is not known because not enough has been made to see it with human eyesight. Based on trends in the Periodic Table it could be a soft, silver colored, very reactive metal like sodium.
History
On February 1, 2004, Nihonium and moscovium were discovered. A team of Russian scientists at Dubna from the Joint Institute for Nuclear Research and American scientists at the Lawrence Livermore National Laboratory first reported the chemical elements.
On September 28, 2004, a team of Japanese scientists said that they had made the element.[9],[10],[11]
In May 2006, in the Joint Institute for Nuclear Research made nihonium using a different method. They found the identity of the last products of the radioactive decay of the nihonium they made.
Name
Ununtrium was a temporary IUPAC systematic element name meaning "one-one-three" in Latin. Scientists from Japan suggested the name japonium (symbol Jp) or rikenium (Rk).[12] However, they picked Nihonium because not only is it discovered in Japan, but it means Japan, too, as Nihon is Japan or Japanese in Japanese.
Nihonium Media
Summary of decay chains passing through isotopes of element 113, ending at mendelevium (element 101) or earlier. The two chains with bold-bordered nuclides were accepted by the JWP as evidence for the discoveries of element 113 and its parents, elements 115 and 117. Data is presented as known in 2015 (before the JWP's conclusions were published).
Kōsuke Morita and Hiroshi Matsumoto, celebrating the naming on 1 December 2016.
A chart of heavy nuclides with their known and predicted half-lives (known nuclides shown with borders). Nihonium (row 113) is expected to be within the "island of stability" (white circle) and thus its nuclei are slightly more stable than would otherwise be predicted; the known nihonium isotopes are too neutron-poor to be within the island.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 1-4020-3555-1.
- ↑ 2.0 2.1 Seaborg, Glenn T. (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- ↑ 3.0 3.1 3.2 Bonchev, Danail; Kamenska, Verginia (1981). "Predicting the Properties of the 113–120 Transactinide Elements". Journal of Physical Chemistry. 85 (9): 1177–1186. doi:10.1021/j150609a021.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Fricke, Burkhard (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. 21: 89–144. doi:10.1007/BFb0116498. Retrieved 4 October 2013.
- ↑ Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements". In Barysz, Maria; Ishikawa, Yasuyuki (eds.). Relativistic Methods for Chemists. Springer. pp. 63–67. doi:10.1007/978-1-4020-9975-5_2. ISBN 978-1-4020-9974-8.
- ↑ Keller, O. L., Jr.; Burnett, J. L.; Carlson, T. A.; Nestor, C. W., Jr. (1969). "Predicted Properties of the Super Heavy Elements. I. Elements 113 and 114, Eka-Thallium and Eka-Lead". The Journal of Physical Chemistry. 74 (5): 1127−1134. doi:10.1021/j100700a029.
- ↑ (2016) "Remarks on the Fission Barriers of SHN and Search for Element 120" in Exotic Nuclei. : 155–164.
- ↑ Hofmann, S.; Heinz, S.; Mann, R.; Maurer, J.; Münzenberg, G.; Antalic, S.; Barth, W.; Burkhard, H. G.; Dahl, L.; Eberhardt, K.; Grzywacz, R.; Hamilton, J. H.; Henderson, R. A.; Kenneally, J. M.; Kindler, B.; Kojouharov, I.; Lang, R.; Lommel, B.; Miernik, K.; Miller, D.; Moody, K. J.; Morita, K.; Nishio, K.; Popeko, A. G.; Roberto, J. B.; Runke, J.; Rykaczewski, K. P.; Saro, S.; Scheidenberger, C.; Schött, H. J.; Shaughnessy, D. A.; Stoyer, M. A.; Thörle-Popiesch, P.; Tinschert, K.; Trautmann, N.; Uusitalo, J.; Yeremin, A. V. (2016). "Review of even element super-heavy nuclei and search for element 120". The European Physics Journal A. 2016 (52). Bibcode:2016EPJA...52..180H. doi:10.1140/epja/i2016-16180-4.
- ↑ Morita et al., Experiment on the Synthesis of Element 113 in the Reaction 209Bi(70Zn, n)278113 Archived 2007-07-05 at the Wayback Machine, J. Phys. Soc. Jpn. Archived 2007-07-01 at the Wayback Machine, Vol. 73, No.10.
- ↑ "press release in Japanese". Archived from the original on 2007-03-01. Retrieved 2019-01-15.
- ↑ Japanese scientists create heaviest ever element
- ↑ Discovering element 113 Archived 2011-08-12 at the Wayback Machine Riken News. Accessed 23 November 2006.
Other websites
- WebElements.com - Nihonium
- Uut and Uup Add Their Atomic Mass to Periodic Table Archived 2006-09-07 at the Wayback Machine
- Apsidium - Nihonium Archived 2008-06-15 at the Wayback Machine
- Discovery of Elements 113 and 115 Archived 2005-06-23 at the Wayback Machine
- Discovery of New Superheavy Elements 113 and 115 Archived 2006-12-30 at the Wayback Machine
- Superheavy elements Archived 2007-04-26 at the Wayback Machine
- Nihonium at The Periodic Table of Videos (University of Nottingham)