Scandium
Scandium is chemical element. Its symbol is Sc. It has an atomic number of 21 on the periodic table.
 | |||||||||||||||
| General properties | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈskændiəm/ | ||||||||||||||
| Appearance | silvery white | ||||||||||||||
| Standard atomic weight (Ar, standard) | 44.955908(5)[1] | ||||||||||||||
| Scandium in the periodic table | |||||||||||||||
| |||||||||||||||
| Atomic number (Z) | 21 | ||||||||||||||
| Group | group n/a | ||||||||||||||
| Period | period {{{period}}} | ||||||||||||||
| Block | [[{{{block}}}-block]] | ||||||||||||||
| Electron configuration | [Ar] 3d1 4s2 | ||||||||||||||
Electrons per shell | 2, 8, 9, 2 | ||||||||||||||
| Physical properties | |||||||||||||||
| Phase at STP | Sc: Solid | ||||||||||||||
| Melting point | 1814 K (1541 °C, 2806 °F) | ||||||||||||||
| Boiling point | 3109 K (2836 °C, 5136 °F) | ||||||||||||||
| Density (near r.t.) | 2.985 g/cm3 | ||||||||||||||
| when liquid (at m.p.) | 2.80 g/cm3 | ||||||||||||||
| Heat of fusion | 14.1 kJ/mol | ||||||||||||||
| Heat of vaporization | 332.7 kJ/mol | ||||||||||||||
| Molar heat capacity | 25.52 J/(mol·K) | ||||||||||||||
Vapor pressure
| |||||||||||||||
| Atomic properties | |||||||||||||||
| Oxidation states | +1,[2] +2,[3] +3 (an amphoteric oxide) | ||||||||||||||
| Electronegativity | Pauling scale: 1.36 | ||||||||||||||
| Ionization energies |
| ||||||||||||||
| Atomic radius | empirical: 162 pm | ||||||||||||||
| Covalent radius | 170±7 pm | ||||||||||||||
| Van der Waals radius | 211 pm | ||||||||||||||
| Spectral lines of scandium | |||||||||||||||
| Other properties | |||||||||||||||
| Natural occurrence | Sc: Primordial | ||||||||||||||
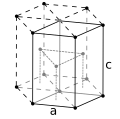
| Crystal structure | hexagonal close-packed (hcp) | ||||||||||||||
| Thermal expansion | α, poly: 10.2 µm/(m·K) (at r.t.) | ||||||||||||||
| Thermal conductivity | 15.8 W/(m·K) | ||||||||||||||
| Electrical resistivity | α, poly: 562 nΩ·m (at r.t., calculated) | ||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||
| Magnetic susceptibility | +315.0×10−6 cm3/mol (292 K)[4] | ||||||||||||||
| Young's modulus | 74.4 GPa | ||||||||||||||
| Shear modulus | 29.1 GPa | ||||||||||||||
| Bulk modulus | 56.6 GPa | ||||||||||||||
| Poisson ratio | 0.279 | ||||||||||||||
| Brinell hardness | 736–1200 MPa | ||||||||||||||
| CAS Number | 7440-20-2 | ||||||||||||||
| History | |||||||||||||||
| Naming | after Scandinavia | ||||||||||||||
| Prediction | Dmitri Mendeleev (1871) | ||||||||||||||
| Discovery and first isolation | Lars Fredrik Nilson (1879) | ||||||||||||||
| |||||||||||||||
Scandium is a metal in a group known as the transition metals. It is also a rare earth metal. What this means is that there is not very much scandium found in the earth. Because of this, the pure metal can be expensive. The pure metal is very reactive, and will react with other elements like oxygen. The metal turns from shiny to gray.
Scandium is not very dangerous because there is not much of it on Earth, so there is not enough of it to harm us. It does not have many uses. Its main use is perhaps as a component in Mercury-vapor lamps. These lamps are used to light stadiums.
Scandium Media
Parts of the MiG-29 are made from Al-Sc alloy.
Related pages
References
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
Further reading
| Library resources about Scandium |
- Scerri, Eric R. (2007). The Periodic System: Its Story and Its Significance. Oxford, UK: Oxford University Press. ISBN 9780195305739. OCLC 62766695.
Other websites
| Wikimedia Commons has media related to Lua error in Module:Commons_link at line 62: attempt to index field 'wikibase' (a nil value).. |
- Scandium at The Periodic Table of Videos (University of Nottingham)
- WebElements.com – Scandium
 Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica (Eleventh ed.). Cambridge University Press.
Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica (Eleventh ed.). Cambridge University Press. {{cite encyclopedia}}: Cite has empty unknown parameter:|coauthors=(help)