Thallium
Thallium is at chemical element, it has symbol Tl and atomic number 81. Its standard atomic weight is 204.4. It is found in Group 15 of the periodic table. Thallium is a soft, heavy and gray metal, but can look red due to oxidation. Thallium and its compounds are extremely toxic, even more than cyanide and arsenic.
 | |||||||||||||||
| General properties | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈθæliəm/ | ||||||||||||||
| Appearance | silvery white | ||||||||||||||
| Standard atomic weight (Ar, standard) | [204.382, 204.385][1] | ||||||||||||||
| Thallium in the periodic table | |||||||||||||||
| |||||||||||||||
| Atomic number (Z) | 81 | ||||||||||||||
| Group | group n/a | ||||||||||||||
| Period | period {{{period}}} | ||||||||||||||
| Block | [[{{{block}}}-block]] | ||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p1 | ||||||||||||||
Electrons per shell | 2, 8, 18, 32, 18, 3 | ||||||||||||||
| Physical properties | |||||||||||||||
| Phase at STP | Tl: Solid | ||||||||||||||
| Melting point | 577 K (304 °C, 579 °F) | ||||||||||||||
| Boiling point | 1746 K (1473 °C, 2683 °F) | ||||||||||||||
| Density (near r.t.) | 11.85 g/cm3 | ||||||||||||||
| when liquid (at m.p.) | 11.22 g/cm3 | ||||||||||||||
| Heat of fusion | 4.14 kJ/mol | ||||||||||||||
| Heat of vaporization | 165 kJ/mol | ||||||||||||||
| Molar heat capacity | 26.32 J/(mol·K) | ||||||||||||||
Vapor pressure
| |||||||||||||||
| Atomic properties | |||||||||||||||
| Oxidation states | −5,[2] −2, −1, +1, +2, +3 (a mildly basic oxide) | ||||||||||||||
| Electronegativity | Pauling scale: 1.62 | ||||||||||||||
| Ionization energies |
| ||||||||||||||
| Atomic radius | empirical: 170 pm | ||||||||||||||
| Covalent radius | 145±7 pm | ||||||||||||||
| Van der Waals radius | 196 pm | ||||||||||||||
| Spectral lines of thallium | |||||||||||||||
| Other properties | |||||||||||||||
| Natural occurrence | Tl: Primordial | ||||||||||||||
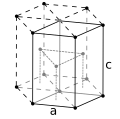
| Crystal structure | hexagonal close-packed (hcp) | ||||||||||||||
| Speed of sound thin rod | 818 m/s (at 20 °C) | ||||||||||||||
| Thermal expansion | 29.9 µm/(m·K) (at 25 °C) | ||||||||||||||
| Thermal conductivity | 46.1 W/(m·K) | ||||||||||||||
| Electrical resistivity | 0.18 µΩ·m (at 20 °C) | ||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||
| Magnetic susceptibility | −50.9×10−6 cm3/mol (298 K)[4] | ||||||||||||||
| Young's modulus | 8 GPa | ||||||||||||||
| Shear modulus | 2.8 GPa | ||||||||||||||
| Bulk modulus | 43 GPa | ||||||||||||||
| Poisson ratio | 0.45 | ||||||||||||||
| Mohs hardness | 1.2 | ||||||||||||||
| Brinell hardness | 26.5–44.7 MPa | ||||||||||||||
| CAS Number | 7440-28-0 | ||||||||||||||
| History | |||||||||||||||
| Naming | after Greek thallos, green shoot or twig | ||||||||||||||
| Discovery | William Crookes (1861) | ||||||||||||||
| First isolation | Claude-Auguste Lamy (1862) | ||||||||||||||
| |||||||||||||||
Properties
Physical properties
Thallium is a soft, malleable, grayish post-transition metal. It can be cut with a knife at room temperature. It melts at a low temperature, 304 °C., which is typical of a post-transition metal. Thallium has 25 known isotopes and two stable (nonradioactive) ones. It is extremely toxic.
Chemical properties
Thallium is a moderately reactive metal. It corrodes easily in air with a color similar to lead. If it is kept in air for a long time, a large amount of thallium(I) oxide will build up. It corrodes in the presence of water to make the hydroxide. It burns with a greenish flame. It reacts with most acids.
Chemical compounds
Thallium makes chemical compounds in two oxidation states: +1 and +3. The +1 state is more common and less reactive. Its chemical compounds are very similar to potassium or silver compounds. It makes a hydroxide that in a strong base when dissolved in water. Most other transition metal and post-tranansition metal hydroxides do not dissolve in water. This reacts with carbon dioxide to make thallium(I) carbonate, which is also water-soluble and very heavy. It is the only heavy metal carbonate that can dissolve in water. Other compounds are similar to silver compounds. Thallium(I) bromide turns yellow when exposed to light, similar to silver(I) bromide. Thallium(I) sulfide is black, similar to silver(I) sulfide. The +3 state compounds are oxidizing agents. The black oxide, thallium(III) oxide and the hydroxide, thallium(III) hydroxide, are the only stable +3 compounds. They break down to oxygen and thallium(I) oxide when heated. Thallium and its compounds are rare because they are toxic and polluting.
+1 compounds
+1 compounds are quite unreactive. It is the more common oxidation state. They are made when thallium dissolves in acids or corrodes in air.
- Thallium(I) bromide, yellowish solid, turns black when in light
- Thallium(I) carbonate, colorless solid, dissolves in water
- Thallium(I) chloride, colorless solid, does not dissolve in water
- Thallium(I) fluoride, colorless solid, dissolves in water
- Thallium(I) hydroxide, yellow solid, strong base, dissolves in water
- Thallium(I) iodide, yellow or red solid, does not dissolve in water
- Thallium(I) oxide, black solid, dissolves in water
- Thallium(I) sulfate, colorless solid, dissolves in water
- Thallium(I) sulfide, black solid, does not dissolve in water
+3 compounds
+3 compounds are oxidizing agents. They are quite rare.
- Thallium(III) halide, the thallium(III) fluoride, chloride, and bromide
- Thallium(III) hydroxide, white solid
- Thallium(III) oxide, white solid, semiconductor
History
Thallium was found by spectroscopy in 1861 by a bright green line in its spectrum. The main use for thallium, rat poison, was banned in many countries in the 1970s. Thallium was also used to poison people, similar to the more popular arsenic.[5]
Occurrence
Thallium is found most in certain clays and granites. It cannot be gotten easily from these, though. Thallium is normally gotten from the waste after other ores like galena are processed.[6] Hutchinsonite is another mineral that has thallium in it.
Preparation
When lead and zinc are taken from their ores, many impurities are left behind. Sulfuric acid is used to dissolve the thallium from it as thallium(I) sulfate. Then the thallium(I) sulfate is electrolyzed to make thallium metal.
Uses
It is used in rat poisons and insecticides. The use of thallium as a poison has been reduced or banned in many countries because these countries think that thallium might cause cancers. It is also used in infrared detectors. It has been used in some murders. Like arsenic, the use of thallium in murders has given it the name "inheritance powder". Thallium compounds are used in glass for infrared light. Thallium was also used to kill skin infections, but it is too toxic to be used for that now. A superconductor that can work at higher temperatures than normal ones do uses thallium. A radioactive thallium isotope was used for nuclear scans. An alloy of thallium and mercury has a low freezing temperature and is a liquid. A very dense solution of a thallium compound was used to test minerals for specific gravity, but it is too toxic for use.
Safety
Thallium is extremely toxic, even touching it is dangerous. Many of its salts easily dissolve. Some are colorless, tasteless, and odorless, but are very toxic. Some think that it is a carcinogen. Thallium can be a pollutant if the thallium waste from metal processing is washed away.
Thallium Media
Crystals of hutchinsonite ((Tl,Pb)2As5S9)
References
- ↑ Meija, J.; Coplen, T. B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; Prohaska, T. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ↑ Dong, Z.-C.; Corbett, J. D. (1996). "Na23K9Tl15.3: An Unusual Zintl Compound Containing Apparent Tl57−, Tl48−, Tl37−, and Tl5− Anions". Inorganic Chemistry. 35 (11): 3107–12. doi:10.1021/ic960014z.
- ↑ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ↑ Hasan, Heather (2009). The Boron Elements: Boron, Aluminum, Gallium, Indium, Thallium. Rosen Publishing Group. p. 14. ISBN 9781435853331.
- ↑ Peter, A; Viraraghavan, T (2005). "Thallium: a review of public health and environmental concerns". Environment International. 31 (4): 493–501. doi:10.1016/j.envint.2004.09.003. PMID 15788190.