Lithium
Lithium (from Greek lithos 'stone') is a soft, silver-white metal with symbol Li. It is the third chemical element in the periodic table. This means that it has 3 protons in its nucleus and 3 electrons around it. Its atomic number is 3. Its mass number is 6.94. It has two common isotopes, 6Li and 7Li. 7Li is more common; 92.5% of lithium is 7Li. Lithium is a soft silvery metal that is very reactive. It is used in lithium batteries and certain medicines.
 Lithium floating in oil | ||||||||||||||||
| General properties | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈlɪθiəm/ | |||||||||||||||
| Appearance | silvery-white | |||||||||||||||
| Standard atomic weight (Ar, standard) | [6.938, 6.997][1] | |||||||||||||||
| Lithium in the periodic table | ||||||||||||||||
| ||||||||||||||||
| Atomic number (Z) | 3 | |||||||||||||||
| Group | group 1 (alkali metals) | |||||||||||||||
| Period | period 2 | |||||||||||||||
| Block | s-block | |||||||||||||||
| Element category | alkali metal | |||||||||||||||
| Electron configuration | [He] 2s1 | |||||||||||||||
Electrons per shell | 2, 1 | |||||||||||||||
| Physical properties | ||||||||||||||||
| Phase at STP | Li: Solid | |||||||||||||||
| Melting point | 453.65 K (180.50 °C, 356.90 °F) | |||||||||||||||
| Boiling point | 1603 K (1330 °C, 2426 °F) | |||||||||||||||
| Density (near r.t.) | 0.534 g/cm3 | |||||||||||||||
| when liquid (at m.p.) | 0.512 g/cm3 | |||||||||||||||
| Critical point | 3220 K, 67 MPa (extrapolated) | |||||||||||||||
| Heat of fusion | 3.00 kJ/mol | |||||||||||||||
| Heat of vaporization | 136 kJ/mol | |||||||||||||||
| Molar heat capacity | 24.860 J/(mol·K) | |||||||||||||||
Vapor pressure
| ||||||||||||||||
| Atomic properties | ||||||||||||||||
| Oxidation states | +1 (a strongly basic oxide) | |||||||||||||||
| Electronegativity | Pauling scale: 0.98 | |||||||||||||||
| Ionization energies |
| |||||||||||||||
| Atomic radius | empirical: 152 pm | |||||||||||||||
| Covalent radius | 128±7 pm | |||||||||||||||
| Van der Waals radius | 182 pm | |||||||||||||||
| Spectral lines of lithium | ||||||||||||||||
| Other properties | ||||||||||||||||
| Natural occurrence | Li: Primordial | |||||||||||||||
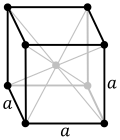
| Crystal structure | body-centered cubic (bcc) | |||||||||||||||
| Speed of sound thin rod | 6000 m/s (at 20 °C) | |||||||||||||||
| Thermal expansion | 46 µm/(m·K) (at 25 °C) | |||||||||||||||
| Thermal conductivity | 84.8 W/(m·K) | |||||||||||||||
| Electrical resistivity | 92.8 nΩ·m (at 20 °C) | |||||||||||||||
| Magnetic ordering | paramagnetic | |||||||||||||||
| Magnetic susceptibility | +14.2·10−6 cm3/mol (298 K)[2] | |||||||||||||||
| Young's modulus | 4.9 GPa | |||||||||||||||
| Shear modulus | 4.2 GPa | |||||||||||||||
| Bulk modulus | 11 GPa | |||||||||||||||
| Mohs hardness | 0.6 | |||||||||||||||
| Brinell hardness | 5 MPa | |||||||||||||||
| CAS Number | 7439-93-2 | |||||||||||||||
| History | ||||||||||||||||
| Discovery | Johan August Arfwedson (1817) | |||||||||||||||
| First isolation | William Thomas Brande (1821) | |||||||||||||||
| Main isotopes of lithium | ||||||||||||||||
| ||||||||||||||||
| 6Li content may be as low as 3.75% in natural samples. 7Li would therefore have a content of up to 96.25%. | ||||||||||||||||
Properties
Physical properties
Lithium is one of the alkali metals. Lithium is a silvery solid metal (when freshly cut). It is very soft. Thus it can be cut easily with a knife. It melts at a low temperature. It is very light, similar to wood. It is the least dense metal and the least dense element in a solid or liquid state. It can hold more heat than any other solid element. It conducts heat and electricity easily.
Chemical properties
It will react with water, giving off hydrogen to form a basic solution (lithium hydroxide). Because of this, lithium must be stored in petroleum jelly. Sodium and potassium can be stored in oil but lithium cannot because it is so light. It will just float on the oil and not be protected by it.
Lithium also reacts with halogens. It can react with nitrogen gas to make lithium nitride. It reacts with air to make a black tarnish and then a white powder of lithium hydroxide and lithium carbonate.
Chemical compounds
- See also: Category:Lithium compounds
Lithium forms chemical compounds with only one oxidation state: +1. Most of them are white and unreactive. They make a bright red color when heated in a flame. They are a little toxic. Most of them dissolve in water. Lithium carbonate is less soluble in water than the other alkali metal carbonates like sodium carbonate.
- Lithium carbonate, used in medicine
- Lithium chloride, colorless crystalline solid, red flame when heated
- Lithium hydroxide, strong base, used to remove carbon dioxide in spaceships
- Lithium nitrate, oxidizing agent
- Lithium nitride, strong base
- Lithium oxide, dissolves in water to make lithium hydroxide
- Lithium peroxide, reacts with water to make oxygen
- Lithium helide, noble gas compound
- Lithium 12-hydroxystearate
- Lithium acetate
- Lithium aluminate
- Lithium aluminium hydride
- Lithium amide
- Lithium aspartate
- Lithium azide
- Lithium beryllide
- Lithium bis(trifluoromethanesulfonyl)imide
- Lithium bis(trimethylsilyl)amide
- Lithium borate
- Lithium bromide
- Lithium carbide
- Lithium chlorate
- Lithium citrate
- Lithium cobalt oxide
- Lithium cyanide
- Lithium diisopropylamide
- Lithium disilicate
- Lithium nitrate.jpg
- Lithium hydroxide.jpg
- Lithium carbonate.jpg
- Lithium chloride.jpg
Occurrence
It does not occur as an element in nature. It only is in the form of lithium compounds. The ocean has a large amount of lithium in it. Certain granites have large amounts of lithium. Most living things have lithium in them. There are some places where much lithium is in the salt. Some silicates have lithium in them.
The largest current find of lithium on Earth may be at the McDermitt Caldera near the Oregon-Nevada border. That's the Greater Yellowstone Ecosystem on this wiki.[3]
History
Lithium (Greek lithos, meaning "stone") was discovered by Johann Arfvedson in 1817. In 1818, Christian Gmelin observed that lithium salts give a bright red color in flame. W.T. Brande and Sir Humphrey Davy later used electrolysis on lithium oxide to isolate the element. Lithium was first used in greases. Then nuclear weapons became a big use of lithium. Lithium was also used to make glass melt easier and make aluminium oxide melt easier in making aluminium. Now lithium is used mainly in batteries.
It was apparently given the name "lithium" because it was discovered from a mineral, while other common alkali metals were first discovered in plant tissue.
Preparation
It is made by getting lithium chloride from pools and springs. The lithium chloride is melted and electrolyzed. This makes liquid lithium and chlorine.
Uses
As an element
Its main use is in batteries. Lithium is used as an anode in the lithium battery. It has more power than batteries with zinc, like alkaline cells. Lithium ion batteries also have lithium in them, though not as an element. It is also used in heat transfer alloys. Lithium is used to make organolithium compounds. They are used for very strong bases.
It is used to make special glasses and ceramics, including the Mount Palomar telescope's 200 inch mirror. Lithium is the lightest known metal and can be alloyed with aluminium, copper, manganese, and cadmium to make strong, lightweight metals for aircraft.
In chemical compounds
Lithium compounds are used in some drugs known as mood stabilizers. Lithium niobate is used in radio transmitters in cell phones. Some lithium compounds are also used in ceramics. Lithium chloride can absorb water from other things. Some lithium compounds are used to make soap and grease. Lithium carbonate is used as a drug to treat manic depression disorder. Lithium carbonate is used for the treatment of bipolar disease and other mental illness conditions.
Organic chemistry
Organolithium compounds are used to make polymers and fine chemicals.[4] Many lithium compounds are used as reagents to make organic compounds. Some lithium compounds like lithium aluminium hydride, lithium triethylborohydride, n-butyllithium and tert-butyllithium are commonly used as very strong bases called superbases.
Other uses
Lithium compounds are used as pyrotechnic colorants and oxidizers in red fireworks and flares.[4][5] Lithium chloride and lithium bromide are used as desiccants for gas streams.[4] Lithium hydroxide and lithium peroxide are used to remove carbon dioxide and purify the air in spacecrafts and submarines.[6] Lithium hydroxide, lithium peroxide and lithium perchlorate are used in oxygen candles that supply submarines with oxygen.[4]
Lithium aluminum hydride can also be used as a solid fuel by itself. Lithium hydride that contains lithium-6 is used in thermonuclear weapons.[7]
Safety
Lithium reacts with water, making irritating smoke and heat. It is not as dangerous as the other alkali metals. Lithium hydroxide is very corrosive. Lithium fires must not be put out with water or an ordinary fire extinguisher. A lithium fire can only be put out with a class D fire extinguisher.
Isotopes
There are 5 isotopes of Lithium having respectively 2, 3, 4, 5 and 6 neutrons in the nucleus. The most common isotope in nature is 3Li7 which makes up 92.58% of the total. The second isotope which is widely available is 3Li6 which makes up 7.42% of the total. The other 3 isotopes are very radioactive and exist in very small quantities. The atomic mass of Lithium is 6.939.
Lithium Media
- Limetal.JPG
Lithium ingots with a thin layer of black nitride tarnish
- Lithium element.jpg
The very light metal lithium in oil
- Nova Centauri 2013 ESO.jpg
Nova Centauri 2013 is the first in which evidence of lithium has been found.
- Arfwedson Johan A.jpg
Johan August Arfwedson is credited with the discovery of lithium in 1817
Hexameric structure of the n-butyllithium fragment in a crystal
Lithium prices
Analyses of the extraction of lithium from seawater, published in 1975
Environmental protests in Belgrade, Serbia, 11 December 2021
Related pages
References
- ↑ Lua error in Module:Citation/CS1/Identifiers at line 630: attempt to index field 'known_free_doi_registrants_t' (a nil value).
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ↑ Peter Zeihan on [1]
- ↑ 4.0 4.1 4.2 4.3 CRC handbook of chemistry and physics, 2000-2001. Lide, David R., 1928- (81st ed.). Boca Raton: CRC Press. 2000. ISBN 0-8493-0481-4. OCLC 44440496.
{{cite book}}: CS1 maint: others (link) - ↑ Wiberg, Egon. (2001). Inorganic chemistry. Wiberg, Nils., Holleman, A. F. (Arnold Frederick), 1859-1953. (1st English ed.). San Diego: Academic Press. ISBN 0-12-352651-5. OCLC 48056955.
- ↑ Air quality in airplane cabins and similar enclosed spaces. Hocking, M. B. (Martin Blake), 1938-. Berlin: Springer. 2005. ISBN 978-3-540-31491-2. OCLC 262680730.
{{cite book}}: CS1 maint: others (link) - ↑ Emsley, John. (2011). Nature's building blocks : an A-Z guide to the elements. Oxford University Press. ISBN 978-0-19-960563-7. OCLC 758646995.